Back
Poster Session C
Epidemiology, health policy and outcomes
Session: (1332–1359) Patient Outcomes, Preferences, and Attitudes Poster II
1339: Impact of the COVID-19 Pandemic on Patients with Rheumatoid Arthritis: Data from the Ontario Best Practices Research Initiative (OBRI)
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Sibel Aydin, MD
University of Ottawa - Ottawa
Ottawa, ON, Canada
Abstract Poster Presenter(s)
Matthew Wong-Pack1, Elliot Hepworth2, Mohammad Movahedi3, Bindee Kuriya1, Janet Pope4, Edward Keystone5, Carter Thorne6, VANDANA AHLUWALIA7, Angela Cesta8, Carol Mously8, Claire Bombardier1, Arthur Lau9 and Sibel Aydin10, 1University of Toronto, Toronto, ON, Canada, 2University of Ottawa, Ottawa, ON, Canada, 3Toronto General Hospital Research Institute, University Health Network, Toronto, ON, Canada, 4University of Western Ontario, London, ON, Canada, 5Keystone Consulting Enterprises Inc., Toronto, ON, Canada, 6Southlake Regional Health Centre, Newmarket, ON, Canada, 7William Osler Health System, Brampton, ON, Canada, 8University Health Network, Toronto, ON, Canada, 9McMaster University, Hamilton, ON, Canada, 10University of Ottawa, Rheumatology, Ottawa, Canada, Ottawa, ON, Canada
Background/Purpose: The COVID-19 Pandemic created challenges for patients with rheumatoid arthritis (RA), including accessing the health care system, transition to unplanned virtual care, reduction in physical activity, and management of disease activity. Our study examined the impact of the pandemic on RA patients reported outcomes (PROs), disease activity (DA) and medication profiles before and after the pandemic.
Methods: The Ontario Best Practices Research Initiative (OBRI) is an observational cohort of adult patients with active RA. We defined two study periods of one year duration each: (1) a pre-COVID-19 pandemic phase (12 months before March 15th, 2020) and (2) a COVID-19 pandemic phase (12 months after March 15th 2020). Patients enrolled in OBRI were included in the analysis if they had at least one visit with a physician or interviewer (virtually) in both study periods. Baseline characteristics, disease activity (DA), as well as other PROs (i.e. Health Assessment Questionnaire Disability Index, Rheumatoid Arthritis Disease Activity Index, EuroQoL- 5 Dimension Questionnaire, and medication use / changes) were included. Paired two-sample t-test and McNamar's test was performed for continuous and categorical variables, respectively.
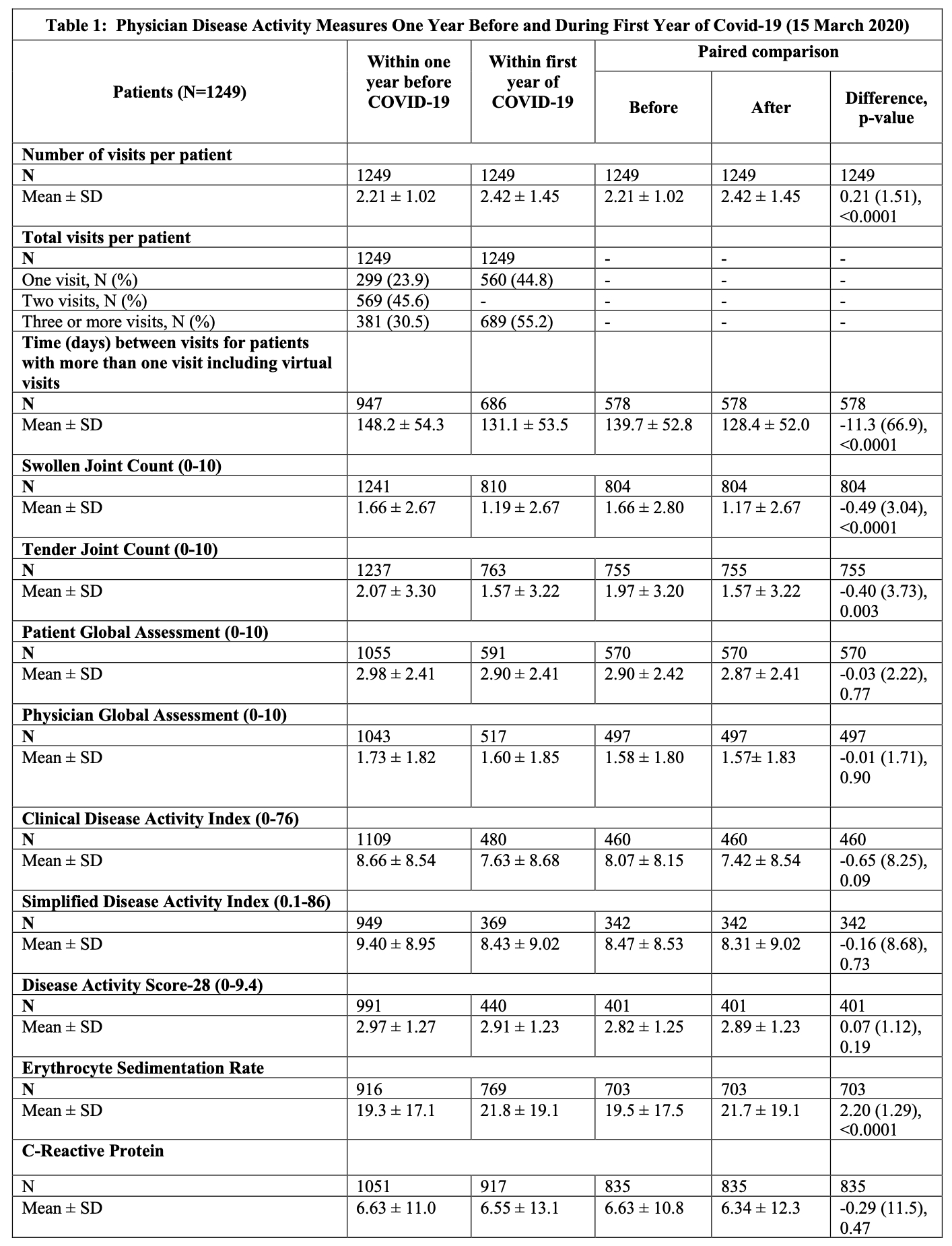
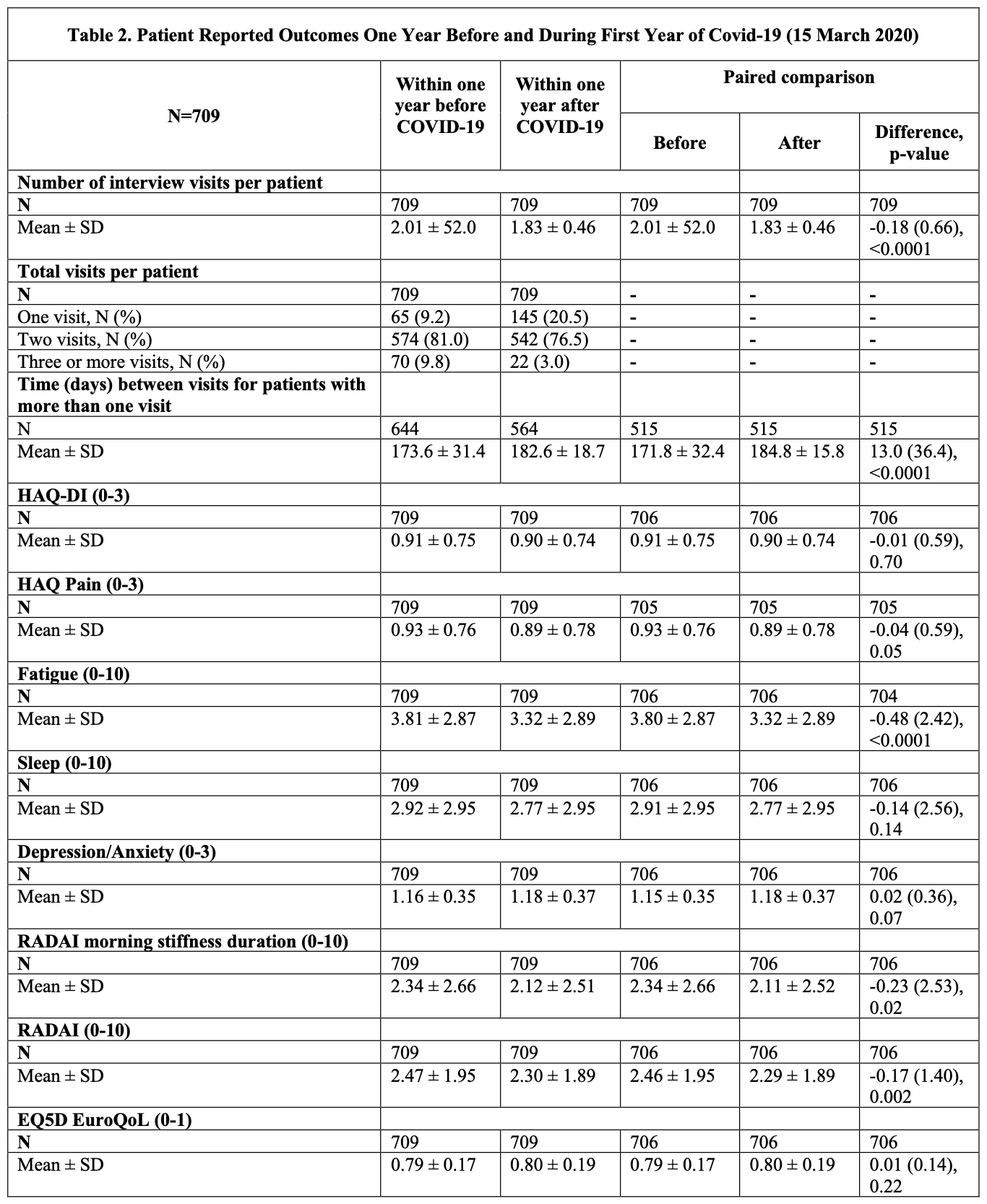
Results: We identified 1508 patients (mean age = 62.7, 79.3% female). Tables 1 and 2 outline changes in disease activity measures and PROs, respectively. During the first year of the pandemic, the number of physician visits per patient increased by a mean of 0.21 visits (SD:1.51) (p < 0.0001). Despite the patient global and physician global assessments and composite DA scores being similar before and after the pandemic, swollen joint count (SJC) and tender joint counts (TJC) showed improvement during the pandemic (differences of -0.49 (3.04), p < 0.0001 and -0.40 (3.73), p < 0.003 respectively). Similar improvements were observed in PROs, such as RADAI (-0.17 (1.40), p = 0.002) and fatigue (-0.48 (2.42), p < 0.0001). There were also statistically significant changes in treatment during the pandemic with decreased biologic (32.6% vs 29.9%; p=0.002) and conventional synthetic DMARDs use (83.3% vs 79.5%; p< 0.0001), while prescription of targeted synthetic DMARDs increased during the pandemic (12.1% vs 15.4%; p< 0.0001).
Conclusion: Disease activity and treatments for RA changed during the pandemic. Improvements in PROs may be explained by changes in patients' lifestyle, such as being able to work from home. Changes in medication profiles may relate to preference of oral agents or those with shorter half-life given the concern of immunosuppression. Future work is needed to determine if these changes persist and what implications they may have beyond the pandemic.
Disclosures: M. Wong-Pack, None; E. Hepworth, None; M. Movahedi, None; B. Kuriya, None; J. Pope, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Eli Lilly, merk, Roche, Seattle Genetics, UCB, Actelion, Amgen, Bayer, Eicos Sciences, Emerald, Gilead, Janssen, Novartis, Pfizer, Sandoz, Sanofi, Boehringer Ingelheim; E. Keystone, AbbVie/Abbott, Amgen, Gilead, Eli Lilly, Merck/MSD, Pfizer, Sanofi, Bristol-Myers Squibb(BMS), Celltrion, Myriad Autoimmune, F. Hoffmann-La Roche Inc, Genentech, Janssen, Sandoz, Samsung Bioepsis, AstraZeneca, Samsung Bioepsis; C. Thorne, AbbVie/Abbott, Amgen, Celgene, CaREBiodam, Centocor, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Medexus/Medac, Merck; V. AHLUWALIA, None; A. Cesta, None; C. Mously, None; C. Bombardier, Amgen, AbbVie, Gilead, Janssen, Eli Lilly, Merck/MSD, Medexus, Medreleaf/Aurora, Novartis, Pfizer, Roche, Sandoz, Sanofi/Genzyme, Samsung, Mylan, GlaxoSmithKlein(GSK); A. Lau, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Merck/MSD, Novartis, Janssen, Pfizer, Roche, Sanofi, UCB; S. Aydin, None.
Background/Purpose: The COVID-19 Pandemic created challenges for patients with rheumatoid arthritis (RA), including accessing the health care system, transition to unplanned virtual care, reduction in physical activity, and management of disease activity. Our study examined the impact of the pandemic on RA patients reported outcomes (PROs), disease activity (DA) and medication profiles before and after the pandemic.
Methods: The Ontario Best Practices Research Initiative (OBRI) is an observational cohort of adult patients with active RA. We defined two study periods of one year duration each: (1) a pre-COVID-19 pandemic phase (12 months before March 15th, 2020) and (2) a COVID-19 pandemic phase (12 months after March 15th 2020). Patients enrolled in OBRI were included in the analysis if they had at least one visit with a physician or interviewer (virtually) in both study periods. Baseline characteristics, disease activity (DA), as well as other PROs (i.e. Health Assessment Questionnaire Disability Index, Rheumatoid Arthritis Disease Activity Index, EuroQoL- 5 Dimension Questionnaire, and medication use / changes) were included. Paired two-sample t-test and McNamar's test was performed for continuous and categorical variables, respectively.
Results: We identified 1508 patients (mean age = 62.7, 79.3% female). Tables 1 and 2 outline changes in disease activity measures and PROs, respectively. During the first year of the pandemic, the number of physician visits per patient increased by a mean of 0.21 visits (SD:1.51) (p < 0.0001). Despite the patient global and physician global assessments and composite DA scores being similar before and after the pandemic, swollen joint count (SJC) and tender joint counts (TJC) showed improvement during the pandemic (differences of -0.49 (3.04), p < 0.0001 and -0.40 (3.73), p < 0.003 respectively). Similar improvements were observed in PROs, such as RADAI (-0.17 (1.40), p = 0.002) and fatigue (-0.48 (2.42), p < 0.0001). There were also statistically significant changes in treatment during the pandemic with decreased biologic (32.6% vs 29.9%; p=0.002) and conventional synthetic DMARDs use (83.3% vs 79.5%; p< 0.0001), while prescription of targeted synthetic DMARDs increased during the pandemic (12.1% vs 15.4%; p< 0.0001).
Conclusion: Disease activity and treatments for RA changed during the pandemic. Improvements in PROs may be explained by changes in patients' lifestyle, such as being able to work from home. Changes in medication profiles may relate to preference of oral agents or those with shorter half-life given the concern of immunosuppression. Future work is needed to determine if these changes persist and what implications they may have beyond the pandemic.


Disclosures: M. Wong-Pack, None; E. Hepworth, None; M. Movahedi, None; B. Kuriya, None; J. Pope, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Eli Lilly, merk, Roche, Seattle Genetics, UCB, Actelion, Amgen, Bayer, Eicos Sciences, Emerald, Gilead, Janssen, Novartis, Pfizer, Sandoz, Sanofi, Boehringer Ingelheim; E. Keystone, AbbVie/Abbott, Amgen, Gilead, Eli Lilly, Merck/MSD, Pfizer, Sanofi, Bristol-Myers Squibb(BMS), Celltrion, Myriad Autoimmune, F. Hoffmann-La Roche Inc, Genentech, Janssen, Sandoz, Samsung Bioepsis, AstraZeneca, Samsung Bioepsis; C. Thorne, AbbVie/Abbott, Amgen, Celgene, CaREBiodam, Centocor, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Medexus/Medac, Merck; V. AHLUWALIA, None; A. Cesta, None; C. Mously, None; C. Bombardier, Amgen, AbbVie, Gilead, Janssen, Eli Lilly, Merck/MSD, Medexus, Medreleaf/Aurora, Novartis, Pfizer, Roche, Sandoz, Sanofi/Genzyme, Samsung, Mylan, GlaxoSmithKlein(GSK); A. Lau, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Merck/MSD, Novartis, Janssen, Pfizer, Roche, Sanofi, UCB; S. Aydin, None.

