Back
Poster Session C
Imaging
Session: (1228–1266) Imaging of Rheumatic Diseases Poster
1244: Incidence of Radiographic Spinal Damage in Psoriatic Arthritis Patients Compared to Spondyloarthritis Patients: Which Patients Are More Affected?
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- Md
Manouk de Hooge, PhD
Ghent University Hospital
Luxembourg, Luxembourg
Abstract Poster Presenter(s)
Manouk de Hooge1, Alla Alexeevna Ishchenko2, Ann-Sophie Kathleen De Craemer3, Serge Steinfeld4, Adrien NZEUSSEU TOUKAP5, Filip Van den bosch6, Dirk Elewaut7, Rik Lories8 and Kurt de Vlam9, 1Ghent University Hospital, Luxembourg, Luxembourg, 2University Hospitals Leuven, Sint-Stevens-Woluwe, Belgium, 3Ghent University Hospital, Gent, Belgium, 4Clinique Saint-Jean, Brussels, Belgium, 5Cliniques Universitaires Saint-Luc, Brussels, Belgium, 6Department of Internal Medicine and Paediatrics, Ghent University and VIB Centre for Inflammation Research, Ghent, Belgium, 7Department of Rheumatology, Ghent University Hospital, Belgium, VIB-UGent Center for Inflammation Research, Ghent University, Heusden, Belgium, 8KU Leuven, Leuven, Belgium, 9University Hospitals Leuven, Leuven, Belgium
Background/Purpose: There is an ongoing debate on axial involvement in psoriatic arthritis (PsA) patients. This study reports the baseline radiographic spinal damage in PsA and SpA patients from 2 prospective multicentre cohort studies in private and academic rheumatology practices.
Methods: PsA patients originated from the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS), a prospective multicentre cohort involving 17 Belgian rheumatology practices. Recruitment was from December 2012 until July 2014. Patients were included when patients fulfilled the Classification criteria for Psoriatic Arthritis (CASPAR). Radiographs of the spine were obtained at baseline and after 2 years. Two calibrated readers evaluated radiographic damage by assessing the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS). When assessing images, readers were blinded for time sequence, clinical data and information from other obtained images (radiographs of the hands and feet).
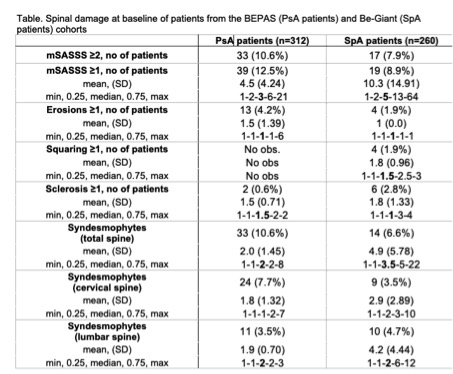
Results: In total 461 patients were included in BEPAS. Mean age 52.79±12.29 years and 43.0% (n=198) were female; average disease duration was 8.5 ± 9.3 years and approximately 34% of the patients reported inflammatory axial pain. From 312 patients spinal radiographs were obtained. At baseline, the vast majority of PsA patients had an mSASSS of 0 (n=273, 87.5%), according to both readers. In 33 PsA patients (10.6%) mSASSS was 2 or more. For SpA patients, percentages were lower but the trend was similar (see figure 1). Though lesser patients showed abnormalities, SpA patients with spinal damage show a higher mSASSS, therefore indicating more spinal damage then the PsA patients (p< 0.05). Both patient groups show some outliers with high mSASSS, increasing the average mSASSS especially in the SpA cohort (mean mSASSS = 10.3±14.91) compared to the median of 5 (IQR 2-13) in the Be-Giant and 3 (IQR 2-6) in the BEPAS cohort. Syndesmophytes are seen in 10.6% and 6.6% of the PsA and SpA patients, respectively. Similar to the mSASSS, SpA patients had more syndesmophytes (mean: 4.9±5.78) compared to PsA patients (mean 2.0±1.45); p< 0.05.PsA patients had more often syndesmophytes located in the cervical spine; 24/33 patients (72.7%) compared to 11/33 (33.3%) patients with lumbar syndesmophytes. Syndesmophyte location was more evenly distributed in SpA patients; cervical 9/14 (64.3.9%) and lumbar 10/14 (71.4%). Percentages exceed 100%, as there were patients with syndesmophytes in both spinal segments.Erosions and especially sclerosis and squaring are uncommon in both patient groups.
Conclusion: Spinal damage is seen in approximately 10% or less of both PsA and SpA patients in these cohorts. SpA patients show higher mSASSS values and more syndesmophytes as compared to the PsA patients. Syndesmophytes in PsA patients are more often located in the cervical spine while the location is more equally distributed in SpA patients.
Disclosures: M. de Hooge, None; A. Ishchenko, None; A. De Craemer, None; S. Steinfeld, None; A. NZEUSSEU TOUKAP, None; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene; D. Elewaut, AbbVie, Eli Lilly, Galapagos, Novartis, UCB Pharma; R. Lories, None; K. de Vlam, UCB, Eli Lilly, Pfizer, AbbVie/Abbott, Merck/MSD, johnson and johnson.
Background/Purpose: There is an ongoing debate on axial involvement in psoriatic arthritis (PsA) patients. This study reports the baseline radiographic spinal damage in PsA and SpA patients from 2 prospective multicentre cohort studies in private and academic rheumatology practices.
Methods: PsA patients originated from the Belgian Epidemiological Psoriatic Arthritis Study (BEPAS), a prospective multicentre cohort involving 17 Belgian rheumatology practices. Recruitment was from December 2012 until July 2014. Patients were included when patients fulfilled the Classification criteria for Psoriatic Arthritis (CASPAR). Radiographs of the spine were obtained at baseline and after 2 years. Two calibrated readers evaluated radiographic damage by assessing the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS). When assessing images, readers were blinded for time sequence, clinical data and information from other obtained images (radiographs of the hands and feet).
Results: In total 461 patients were included in BEPAS. Mean age 52.79±12.29 years and 43.0% (n=198) were female; average disease duration was 8.5 ± 9.3 years and approximately 34% of the patients reported inflammatory axial pain. From 312 patients spinal radiographs were obtained. At baseline, the vast majority of PsA patients had an mSASSS of 0 (n=273, 87.5%), according to both readers. In 33 PsA patients (10.6%) mSASSS was 2 or more. For SpA patients, percentages were lower but the trend was similar (see figure 1). Though lesser patients showed abnormalities, SpA patients with spinal damage show a higher mSASSS, therefore indicating more spinal damage then the PsA patients (p< 0.05). Both patient groups show some outliers with high mSASSS, increasing the average mSASSS especially in the SpA cohort (mean mSASSS = 10.3±14.91) compared to the median of 5 (IQR 2-13) in the Be-Giant and 3 (IQR 2-6) in the BEPAS cohort. Syndesmophytes are seen in 10.6% and 6.6% of the PsA and SpA patients, respectively. Similar to the mSASSS, SpA patients had more syndesmophytes (mean: 4.9±5.78) compared to PsA patients (mean 2.0±1.45); p< 0.05.PsA patients had more often syndesmophytes located in the cervical spine; 24/33 patients (72.7%) compared to 11/33 (33.3%) patients with lumbar syndesmophytes. Syndesmophyte location was more evenly distributed in SpA patients; cervical 9/14 (64.3.9%) and lumbar 10/14 (71.4%). Percentages exceed 100%, as there were patients with syndesmophytes in both spinal segments.Erosions and especially sclerosis and squaring are uncommon in both patient groups.
Conclusion: Spinal damage is seen in approximately 10% or less of both PsA and SpA patients in these cohorts. SpA patients show higher mSASSS values and more syndesmophytes as compared to the PsA patients. Syndesmophytes in PsA patients are more often located in the cervical spine while the location is more equally distributed in SpA patients.

Disclosures: M. de Hooge, None; A. Ishchenko, None; A. De Craemer, None; S. Steinfeld, None; A. NZEUSSEU TOUKAP, None; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene; D. Elewaut, AbbVie, Eli Lilly, Galapagos, Novartis, UCB Pharma; R. Lories, None; K. de Vlam, UCB, Eli Lilly, Pfizer, AbbVie/Abbott, Merck/MSD, johnson and johnson.

