Back
Poster Session C
Metabolic bone disease
Session: (1304–1331) Osteoporosis and Metabolic Bone Disease – Basic and Clinical Science Poster
1318: Low-Dose Prednisolone (≤5 Mg/d) Is Not Associated with Deleterious Effects on Bone Mineral Density: Baseline Findings in a Cohort of Rheumatic Disease Patients with Prior Glucocorticoid Exposure
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- EW
Edgar Wiebe, MD
Charité University Medicine Berlin
Berlin, Germany
Abstract Poster Presenter(s)
Edgar Wiebe1, Dörte Huscher2, Desirée Schaumburg1, Andriko Palmowski1, Sandra Hermann1, Thomas Buttgereit3, Robert Biesen4, Gerd Burmester5, Yannick Palmowski6, Maarten Boers7, John Stone8, Christian Dejaco9 and Frank Buttgereit10, 1Department of Rheumatology and Clinical Immunology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany, 2Institute of Biometry and Clinical Epidemiology, and Berlin Institute of Health, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany, 3Department of Dermatology, Venerology and Allergology, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany, 4Charité Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany, 5Charité University Medicine Berlin, Berlin, Germany, 6Spine Department, Center for Musculoskeletal Surgery, Charité – Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin, Berlin, Germany, 7Amsterdam UMC, Vrije Universiteit, Amsterdam, Netherlands, 8Massachusetts General Hospital Rheumatology Unit, Harvard Medical School, Boston, MA, 9Department of Rheumatology, Hospital of Brunico (SABES-ASDAA), Brunico, Italy, and Department of Rheumatology and Immunology, Medical University of Graz, Graz, Austria, 10Charité Universitätsmedizin Berlin, Corporate Member of Freie Universität Berlin and Humboldt-Universität zu Berlin / DRFZ Berlin, Berlin, Germany
Background/Purpose: Inflammatory rheumatic and musculoskeletal diseases (iRMD) are associated with increased systemic bone loss that is mediated by chronic inflammation, treatment with glucocorticoids (GC), and other factors, leading to an increased risk of osteoporosis and fragility fractures. Our objective was to analyze the impact of variables that influence osteoporosis in patients with iRMD treated with GC.
Methods: Rh-GIOP is a prospective observational cohort study investigating bone health in consecutive patients with iRMD and current or prior GC treatment. We present an analysis of the patients' baseline data here. Bone mineral density (BMD) measured by dual X-ray absorptiometry (DXA) was the primary outcome. Multivariable linear regression models were performed to identify variables associated with BMD. The focus lay on analyzing the impact of current GC dose on BMD as well as the impact of the interaction between GC dose and disease activity.
Results: Data from 1,066 patients with iRMD were analyzed. GC doses of < 5 mg prednisone equivalent per day, cumulative dose and duration of GC therapy were not associated with negative effects on BMD. Dosages ≥5 mg/d lost their negative association with BMD after adjustment for confounders. When sub-analyzing patients with exactly 5 mg/d, no negative effect was seen. For patients with rheumatoid arthritis (RA), GC doses >7.5 mg per day showed a negative association with BMD overall, but this effect seemed to be specific only to patients with moderate or high disease activity (DAS28-CRP >3.2).
Conclusion: Glucocorticoids ≤5 mg/d did not seem to be associated with a reduction of BMD in patients with iRMD and current or prior exposure to GC. This is most likely due to the dampening of inflammation by GC, which exerts a mitigating effect on the risk of osteoporosis. We conclude that in patients with iRMD a) both optimal disease control, optimum glucocorticoid doses and sufficient osteoporosis treatment measures (such as normal vitamin D levels, appropriate use of anti-osteoporosis drugs) are essential for bone protection, and b) low GC dosages (≤ 5 mg/d), aimed at achieving sustained remission or low disease activity, are likely to be safe in terms of bone health.
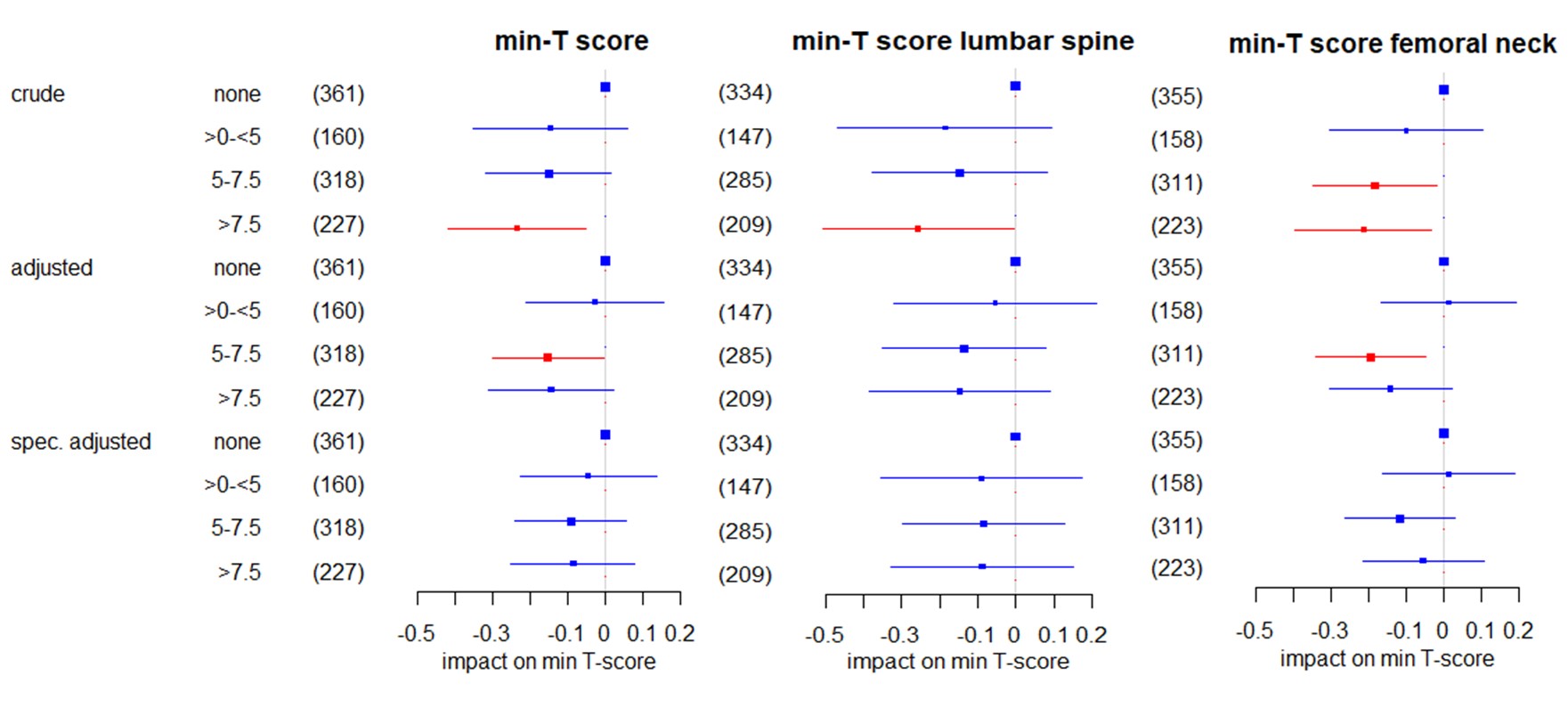 Figure 1: Impact of the current GC dose on the lowest (min-) T-score in all patients in linear regression using: 1) a crude model only including GC categories; 2) a multivariable model adjusted for age, sex, menopause, BMI, alkaline phosphatase, disease duration, bisphosphonates and denosumab; and, 3) a multivariable model specifically adjusted for the variables that emerged in the data mining process and were confirmed with backward selection for the respective T score. The regression coefficients β and respective 95% confidence intervals are shown. Significant coefficients are highlighted in red. The size of the boxes indicates the case numbers, also shown in brackets, of the respective groups; these are the rounded pooled case numbers of the 10 imputed data sets. For “no GC” as the reference group no coefficient was estimated.
Figure 1: Impact of the current GC dose on the lowest (min-) T-score in all patients in linear regression using: 1) a crude model only including GC categories; 2) a multivariable model adjusted for age, sex, menopause, BMI, alkaline phosphatase, disease duration, bisphosphonates and denosumab; and, 3) a multivariable model specifically adjusted for the variables that emerged in the data mining process and were confirmed with backward selection for the respective T score. The regression coefficients β and respective 95% confidence intervals are shown. Significant coefficients are highlighted in red. The size of the boxes indicates the case numbers, also shown in brackets, of the respective groups; these are the rounded pooled case numbers of the 10 imputed data sets. For “no GC” as the reference group no coefficient was estimated.
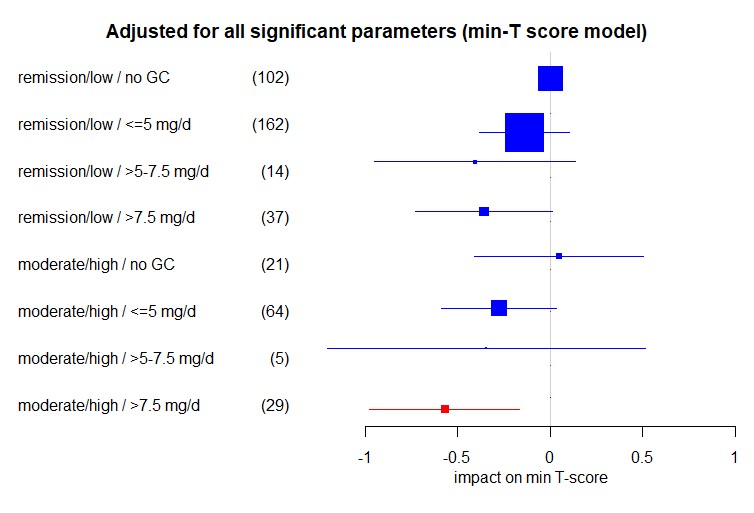 Impact of the interaction of disease activity and current GC dose on the lowest (minimum = min.) T-Score in RA patients in multivariable linear regression adjusted for age, menopause, BMI, alkaline phosphatase, bisphosphonates, disease duration, denosumab, and male sex. Shown are regression coefficients β and respective 95% confidence intervals. Significant coefficients are highlighted in red. The size of the boxes indicates the case numbers, also shown in brackets, of the respective groups; these are the rounded pooled case numbers of the 10 imputed data sets. For “remission/low / no GC” as the reference group no coefficient was estimated.
Impact of the interaction of disease activity and current GC dose on the lowest (minimum = min.) T-Score in RA patients in multivariable linear regression adjusted for age, menopause, BMI, alkaline phosphatase, bisphosphonates, disease duration, denosumab, and male sex. Shown are regression coefficients β and respective 95% confidence intervals. Significant coefficients are highlighted in red. The size of the boxes indicates the case numbers, also shown in brackets, of the respective groups; these are the rounded pooled case numbers of the 10 imputed data sets. For “remission/low / no GC” as the reference group no coefficient was estimated.
Disclosures: E. Wiebe, Medac, Novartis; D. Huscher, Shire; D. Schaumburg, None; A. Palmowski, None; S. Hermann, AbbVie; T. Buttgereit, Roche, Novartis, Sanofi, GlaxoSmithKlein(GSK); R. Biesen, Novartis; G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc.; Y. Palmowski, None; M. Boers, Novartis; J. Stone, Horizon Theraputics, Sanofi, Amgen, Argenx, Bristol-Myers Squibb(BMS), Chemocentryx, Kyverna, Novartis, Palleon Pharmaceuticals, PPD, Q32, Star Therapeutics, Roche, Mirabio, Spruce Biosciences, Steritas, Zenas; C. Dejaco, Abbvie, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Galapagos and Sanofi; F. Buttgereit, Horizon Therapeutics, Roche, Abbvie, AstraZeneca, Gruenenthal, Mundipharma, Pfizer.
Background/Purpose: Inflammatory rheumatic and musculoskeletal diseases (iRMD) are associated with increased systemic bone loss that is mediated by chronic inflammation, treatment with glucocorticoids (GC), and other factors, leading to an increased risk of osteoporosis and fragility fractures. Our objective was to analyze the impact of variables that influence osteoporosis in patients with iRMD treated with GC.
Methods: Rh-GIOP is a prospective observational cohort study investigating bone health in consecutive patients with iRMD and current or prior GC treatment. We present an analysis of the patients' baseline data here. Bone mineral density (BMD) measured by dual X-ray absorptiometry (DXA) was the primary outcome. Multivariable linear regression models were performed to identify variables associated with BMD. The focus lay on analyzing the impact of current GC dose on BMD as well as the impact of the interaction between GC dose and disease activity.
Results: Data from 1,066 patients with iRMD were analyzed. GC doses of < 5 mg prednisone equivalent per day, cumulative dose and duration of GC therapy were not associated with negative effects on BMD. Dosages ≥5 mg/d lost their negative association with BMD after adjustment for confounders. When sub-analyzing patients with exactly 5 mg/d, no negative effect was seen. For patients with rheumatoid arthritis (RA), GC doses >7.5 mg per day showed a negative association with BMD overall, but this effect seemed to be specific only to patients with moderate or high disease activity (DAS28-CRP >3.2).
Conclusion: Glucocorticoids ≤5 mg/d did not seem to be associated with a reduction of BMD in patients with iRMD and current or prior exposure to GC. This is most likely due to the dampening of inflammation by GC, which exerts a mitigating effect on the risk of osteoporosis. We conclude that in patients with iRMD a) both optimal disease control, optimum glucocorticoid doses and sufficient osteoporosis treatment measures (such as normal vitamin D levels, appropriate use of anti-osteoporosis drugs) are essential for bone protection, and b) low GC dosages (≤ 5 mg/d), aimed at achieving sustained remission or low disease activity, are likely to be safe in terms of bone health.
 Figure 1: Impact of the current GC dose on the lowest (min-) T-score in all patients in linear regression using: 1) a crude model only including GC categories; 2) a multivariable model adjusted for age, sex, menopause, BMI, alkaline phosphatase, disease duration, bisphosphonates and denosumab; and, 3) a multivariable model specifically adjusted for the variables that emerged in the data mining process and were confirmed with backward selection for the respective T score. The regression coefficients β and respective 95% confidence intervals are shown. Significant coefficients are highlighted in red. The size of the boxes indicates the case numbers, also shown in brackets, of the respective groups; these are the rounded pooled case numbers of the 10 imputed data sets. For “no GC” as the reference group no coefficient was estimated.
Figure 1: Impact of the current GC dose on the lowest (min-) T-score in all patients in linear regression using: 1) a crude model only including GC categories; 2) a multivariable model adjusted for age, sex, menopause, BMI, alkaline phosphatase, disease duration, bisphosphonates and denosumab; and, 3) a multivariable model specifically adjusted for the variables that emerged in the data mining process and were confirmed with backward selection for the respective T score. The regression coefficients β and respective 95% confidence intervals are shown. Significant coefficients are highlighted in red. The size of the boxes indicates the case numbers, also shown in brackets, of the respective groups; these are the rounded pooled case numbers of the 10 imputed data sets. For “no GC” as the reference group no coefficient was estimated. Impact of the interaction of disease activity and current GC dose on the lowest (minimum = min.) T-Score in RA patients in multivariable linear regression adjusted for age, menopause, BMI, alkaline phosphatase, bisphosphonates, disease duration, denosumab, and male sex. Shown are regression coefficients β and respective 95% confidence intervals. Significant coefficients are highlighted in red. The size of the boxes indicates the case numbers, also shown in brackets, of the respective groups; these are the rounded pooled case numbers of the 10 imputed data sets. For “remission/low / no GC” as the reference group no coefficient was estimated.
Impact of the interaction of disease activity and current GC dose on the lowest (minimum = min.) T-Score in RA patients in multivariable linear regression adjusted for age, menopause, BMI, alkaline phosphatase, bisphosphonates, disease duration, denosumab, and male sex. Shown are regression coefficients β and respective 95% confidence intervals. Significant coefficients are highlighted in red. The size of the boxes indicates the case numbers, also shown in brackets, of the respective groups; these are the rounded pooled case numbers of the 10 imputed data sets. For “remission/low / no GC” as the reference group no coefficient was estimated.Disclosures: E. Wiebe, Medac, Novartis; D. Huscher, Shire; D. Schaumburg, None; A. Palmowski, None; S. Hermann, AbbVie; T. Buttgereit, Roche, Novartis, Sanofi, GlaxoSmithKlein(GSK); R. Biesen, Novartis; G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc.; Y. Palmowski, None; M. Boers, Novartis; J. Stone, Horizon Theraputics, Sanofi, Amgen, Argenx, Bristol-Myers Squibb(BMS), Chemocentryx, Kyverna, Novartis, Palleon Pharmaceuticals, PPD, Q32, Star Therapeutics, Roche, Mirabio, Spruce Biosciences, Steritas, Zenas; C. Dejaco, Abbvie, Eli Lilly, Janssen, Novartis, Pfizer, Roche, Galapagos and Sanofi; F. Buttgereit, Horizon Therapeutics, Roche, Abbvie, AstraZeneca, Gruenenthal, Mundipharma, Pfizer.

