Back
Poster Session C
Pain syndromes, fibromyalgia and regional musculoskeletal disorders
Session: (1215–1227) Fibromyalgia and Other Clinical Pain Syndromes Poster
1221: Multi-Center Validation of Cell-Bound Complement Activation Products and a Multianalyte Assay Panel Distinguishing Systemic Lupus Erythematosus from Primary Fibromyalgia
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MR
Mark Rudolph, PhD
Exagen, Inc
Vista, CA, United States
Abstract Poster Presenter(s)
Mark Rudolph1, Scott Rey1, Alan Kivitz2, Stuart Silverman3, Roland Staud4 and Roberta Alexander1, 1Exagen, Inc., Vista, CA, 2Department of Rheumatology, Altoona Center for Clinical Research, Duncansville, PA, 3Cedars-Sinai Medical Center, Los Angeles, CA, 4University of Florida, Gainesville, FL
Background/Purpose: A 2016 article in Lupus Science & Medicine (Wallace et al. 2016) described the ability of cell-bound complement activation products (CB-CAPs) on their own-- and incorporated into a multianalyte assay panel (MAP)-- in differentiating between systemic lupus erythematosus (SLE) and primary fibromyalgia syndrome (FMS). This study expanded to a multi-center investigation of the MAP in SLE, FMS, normal healthy volunteers (NHV), and additional rheumatological conditions including rheumatoid arthritis (RA), chronic localized pain (CLP), and Sjogren’s Syndrome (SjS).
Methods: Study participants were recruited across 10 US sites. Patients with RA, SLE, SjS, and FMS required a clinical diagnosis and fulfillment of respective criteria (2010 ACR classification criteria for RA; 1997 ACR, 2012 SLICC or 2019 EULAR/ACR criteria for SLE; 2016 ACR/EULAR classification criteria for SjS; 2016 diagnostic/classification criteria for FMS). NHV and CLP participants were required to have a polysymptomatic distress score of ≤ 10 at enrollment. Erythrocyte (EC4d), B-cell (BC4d), and platelet (PC4d) CB-CAPs were measured by flow cytometry in a CAP/CLIA certified laboratory. Positive results are defined as EC4d > 14 net mean fluorescence intensity (MFI), BC4d > 60 net MFI, and PC4d > 20 net MFI. Additionally, anti-nuclear antibodies (ANA), anti-double stranded DNA antibodies (dsDNA; confirmed by Crithidia luciliae), and turbidimetric complement C3 and C4 were measured for each participant. The MAP includes EC4d, BC4d, anti-dsDNA, ANA, anti-Smith antibodies, and five specificity component auto-antibodies (Putterman et al., 2014).
Results: ANA positivity was observed in most of the SLE and SjS participants, but in less than 20% of all other patient groups (Table 1). As in the 2016 publication, participants were divided by ANA positivity prior to analysis of quantitative and qualitative laboratory results. Comparisons between SLE and FMS are very consistent between the previous study and this multi-center effort (Table 2, Wallace et al. 2016). Further, we report here additional results, including MAP score and PC4d, across participant groups not included in the original publication (Table 2).
Conclusion: As designed, the MAP is a highly effective tool to aid the diagnosis of SLE. Indeed, specificity components such as anti-CCP for RA help to offset the instances of CB-CAPs or ANA positivity among different rheumatological conditions. Importantly, of the 157 FMS patients, only two presented with a positive MAP score, and both were low positive (score 0.3 and 0.5, while the median score in the SLE cohort was 2.05). This confirms, across a multi-site study, the ability of our MAP to effectively rule out FMS and other conditions among symptomatic patients referred to rheumatologists.
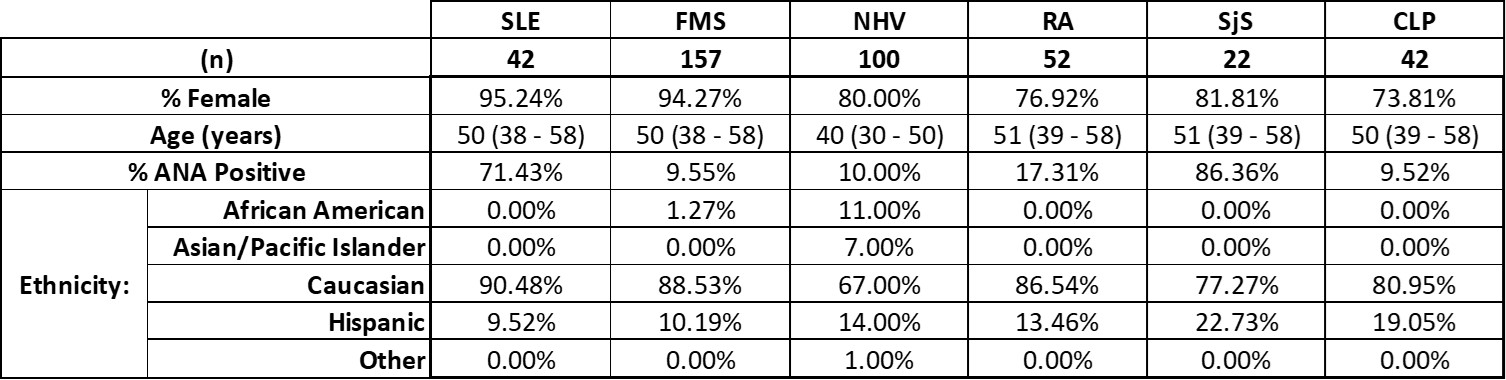 Table 1. Demographic characteristics of the study cohort, divided by disease group.
Table 1. Demographic characteristics of the study cohort, divided by disease group.
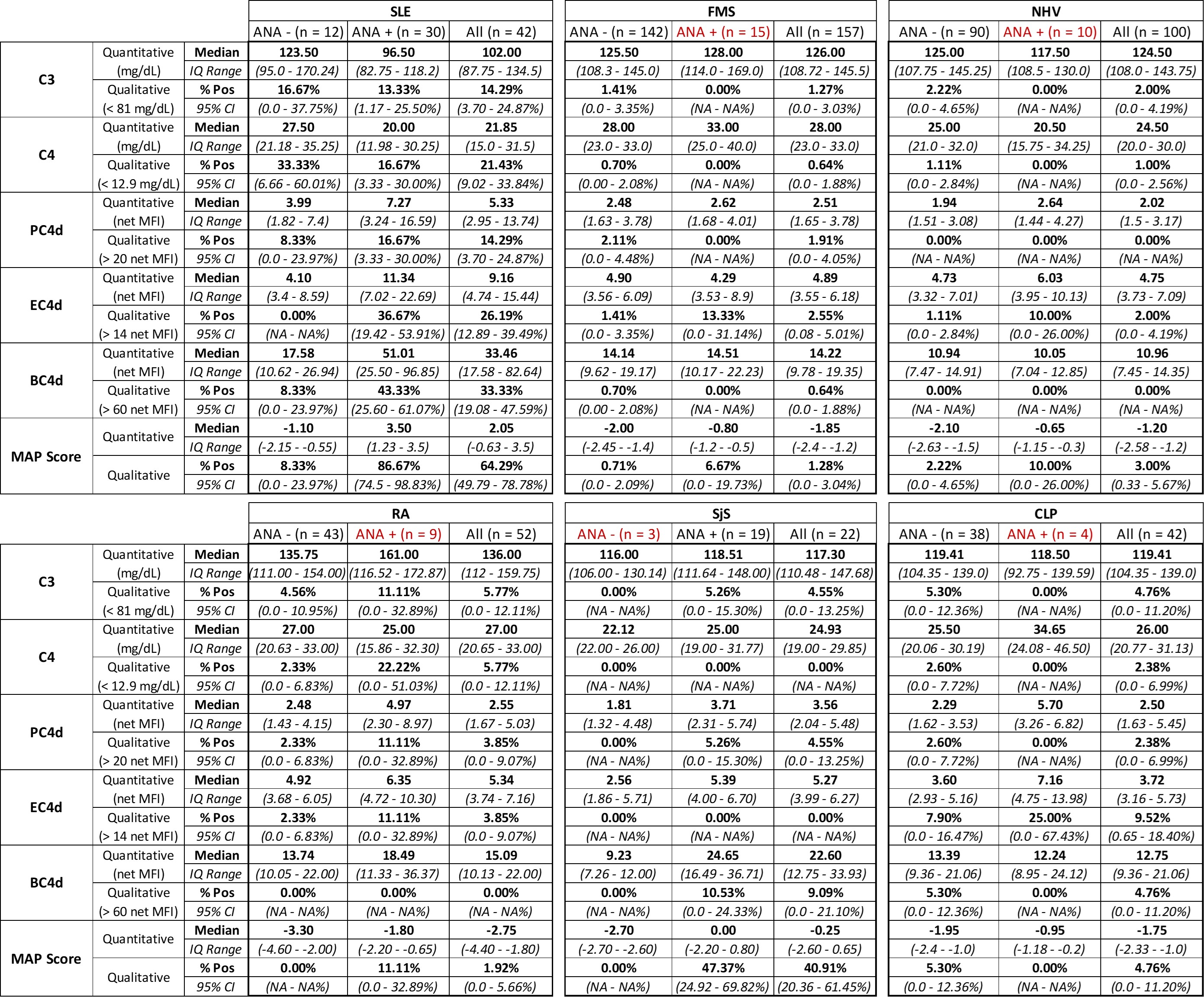 Table 2. Absolute values (quantitative) and positivity rate (qualitative) of serum complement protein C3 and C4, CB-CAPs (EC4d, BC4d, and PC4d), and MAP score. Patient groups are divided by disease group, and subdivided into ANA negative (ANA -), ANA positive (ANA +), and total regardless of ANA status (All). Quantitative results are presented as a median value with interquartile ranges. Qualitative results are presented as percent positivity with 95% confidence intervals.
Table 2. Absolute values (quantitative) and positivity rate (qualitative) of serum complement protein C3 and C4, CB-CAPs (EC4d, BC4d, and PC4d), and MAP score. Patient groups are divided by disease group, and subdivided into ANA negative (ANA -), ANA positive (ANA +), and total regardless of ANA status (All). Quantitative results are presented as a median value with interquartile ranges. Qualitative results are presented as percent positivity with 95% confidence intervals.
Disclosures: M. Rudolph, Exagen, Inc; S. Rey, Exagen Inc.; A. Kivitz, Amgen, Boehringer-Ingelheim, Janssen, Gilead, GlaxoSmithKlein (GSK), Novartis, Pfizer, Sanofi, Flexion, Eli Lilly, Genentech, UCB, AbbVie, Merck, ECOR1 CAPITAL, LLC, Chemocentryx, Regenerson, Grunenthal, Bendcare, Horizon; S. Silverman, Exagen, Inc; R. Staud, None; R. Alexander, Exagen Inc..
Background/Purpose: A 2016 article in Lupus Science & Medicine (Wallace et al. 2016) described the ability of cell-bound complement activation products (CB-CAPs) on their own-- and incorporated into a multianalyte assay panel (MAP)-- in differentiating between systemic lupus erythematosus (SLE) and primary fibromyalgia syndrome (FMS). This study expanded to a multi-center investigation of the MAP in SLE, FMS, normal healthy volunteers (NHV), and additional rheumatological conditions including rheumatoid arthritis (RA), chronic localized pain (CLP), and Sjogren’s Syndrome (SjS).
Methods: Study participants were recruited across 10 US sites. Patients with RA, SLE, SjS, and FMS required a clinical diagnosis and fulfillment of respective criteria (2010 ACR classification criteria for RA; 1997 ACR, 2012 SLICC or 2019 EULAR/ACR criteria for SLE; 2016 ACR/EULAR classification criteria for SjS; 2016 diagnostic/classification criteria for FMS). NHV and CLP participants were required to have a polysymptomatic distress score of ≤ 10 at enrollment. Erythrocyte (EC4d), B-cell (BC4d), and platelet (PC4d) CB-CAPs were measured by flow cytometry in a CAP/CLIA certified laboratory. Positive results are defined as EC4d > 14 net mean fluorescence intensity (MFI), BC4d > 60 net MFI, and PC4d > 20 net MFI. Additionally, anti-nuclear antibodies (ANA), anti-double stranded DNA antibodies (dsDNA; confirmed by Crithidia luciliae), and turbidimetric complement C3 and C4 were measured for each participant. The MAP includes EC4d, BC4d, anti-dsDNA, ANA, anti-Smith antibodies, and five specificity component auto-antibodies (Putterman et al., 2014).
Results: ANA positivity was observed in most of the SLE and SjS participants, but in less than 20% of all other patient groups (Table 1). As in the 2016 publication, participants were divided by ANA positivity prior to analysis of quantitative and qualitative laboratory results. Comparisons between SLE and FMS are very consistent between the previous study and this multi-center effort (Table 2, Wallace et al. 2016). Further, we report here additional results, including MAP score and PC4d, across participant groups not included in the original publication (Table 2).
Conclusion: As designed, the MAP is a highly effective tool to aid the diagnosis of SLE. Indeed, specificity components such as anti-CCP for RA help to offset the instances of CB-CAPs or ANA positivity among different rheumatological conditions. Importantly, of the 157 FMS patients, only two presented with a positive MAP score, and both were low positive (score 0.3 and 0.5, while the median score in the SLE cohort was 2.05). This confirms, across a multi-site study, the ability of our MAP to effectively rule out FMS and other conditions among symptomatic patients referred to rheumatologists.
 Table 1. Demographic characteristics of the study cohort, divided by disease group.
Table 1. Demographic characteristics of the study cohort, divided by disease group. Table 2. Absolute values (quantitative) and positivity rate (qualitative) of serum complement protein C3 and C4, CB-CAPs (EC4d, BC4d, and PC4d), and MAP score. Patient groups are divided by disease group, and subdivided into ANA negative (ANA -), ANA positive (ANA +), and total regardless of ANA status (All). Quantitative results are presented as a median value with interquartile ranges. Qualitative results are presented as percent positivity with 95% confidence intervals.
Table 2. Absolute values (quantitative) and positivity rate (qualitative) of serum complement protein C3 and C4, CB-CAPs (EC4d, BC4d, and PC4d), and MAP score. Patient groups are divided by disease group, and subdivided into ANA negative (ANA -), ANA positive (ANA +), and total regardless of ANA status (All). Quantitative results are presented as a median value with interquartile ranges. Qualitative results are presented as percent positivity with 95% confidence intervals. Disclosures: M. Rudolph, Exagen, Inc; S. Rey, Exagen Inc.; A. Kivitz, Amgen, Boehringer-Ingelheim, Janssen, Gilead, GlaxoSmithKlein (GSK), Novartis, Pfizer, Sanofi, Flexion, Eli Lilly, Genentech, UCB, AbbVie, Merck, ECOR1 CAPITAL, LLC, Chemocentryx, Regenerson, Grunenthal, Bendcare, Horizon; S. Silverman, Exagen, Inc; R. Staud, None; R. Alexander, Exagen Inc..

