Back
Poster Session C
Epidemiology, health policy and outcomes
Session: (1332–1359) Patient Outcomes, Preferences, and Attitudes Poster II
1356: Patient-perceived Disease Burden and Self-reported Medication Adherence in Systemic Autoimmune Diseases: A Monocentric Observational Study
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- CM
Camille Mettler, MD
APHP, Service de Médecine Interne, Hôpital Cochin
Paris, France
Abstract Poster Presenter(s)
Camille Mettler1, Veronique Le Guern2, Paul Legendre1, Caroline Morbieu1, Tali-Anne Swebel1, Xavier Puéchal3, Pascal Cohen3, Hicham Kardaoui1, Alexandre Moores1, Luc Mouthon3, Nathalie Costedoat-Chalumeau4 and Benjamin Terrier3, 1Department of Internal Medicine, National Referral Center for Rare Systemic and Autoimmune Diseases, Cochin Hospital, Paris, France, 2Hôpital Cochin, Paris, France, 3National Referral Center for Rare Systemic Autoimmune Diseases, Cochin Hospital, Paris, France, 4Inserm DR Paris 5, Paris, France
Background/Purpose: Systemic autoimmune diseases (AID) are chronic conditions with significant burden related to the disease itself, its management, and treatments. Burden refers to the workload imposed by disease and healthcare on patients. Furthermore, management of these diseases may be impacted by non-adherence to treatment which can be influenced by many determinants. We aimed to describe factors associated with disease burden and medication adherence in patients with systemic AID and to assess the relationship between these two aspects.
Methods: We performed an observational study using an anonymous questionnaire among patients followed-up in our outpatient AID reference center. Disease burden was estimated by a validated score composed of 13 items, ranging from 0 (no burden) to 10 (maximum burden) (1,2). High burden was defined as greater than its median. For each treatment, medication adherence was assessed using a validated questionnaire to classify adherence into 6 levels (1: high level of adherence; 6: no treatment at all) (3). Logistic regression models and Spearman correlation test were performed.
Results: We included 467 patients, 73% were women and median age was 47 years (IQR 35.5-63). Main AID were systemic vasculitis in 46% and systemic lupus erythematosus, Sjögren's syndrome and/or antiphospholipid syndrome in 32%. Median disease duration was 7 years (IQR 3-13). Main treatments used were glucocorticoids (GCs) and hydroxychloroquine (HCQ) in 54% and 30% of patients, respectively. Median treatment burden score was 29/130 (IQR 13-47) (Figure 1). In univariate analysis, factors associated with high burden were age < 40 years, high number of physicians involved in disease management, number of drugs per day, number of daily tablets, active disease, GCs, azathioprine, and mycophenolate mofetil (MMF) use, and antidepressant treatment. In multivariate analysis, determinants that remained independently associated with high burden were age < 40 years (OR 2.25; 95% CI [1.27-4.03]), active disease (OR 4.24; 95% CI [2.53-7.25]), and GCs use (OR 2.21; 95% CI [1.38-3.55]). Median adherence to GCs and methotrexate were good (2/6), whereas adherence to azathioprine, HCQ and MMF were moderate (3/6) (Figure 2). GCs and azathioprine adherence scores were correlated with the importance attributed to treatments (p=0.014 and p< 0.001, respectively), whereas this association was not found for other treatments. Interestingly, disease burden and medication adherence scores were not significantly correlated in our cohort
Conclusion: High disease burden was significantly associated with younger age, active disease and GCs use, suggesting the need for special attention in these patients. Medication adherence was not always correlated with the importance attributed to it, nor with treatment burden. Studies evaluating the impact of specific actions would be of interest to decrease patient burden and to improve medication adherence.
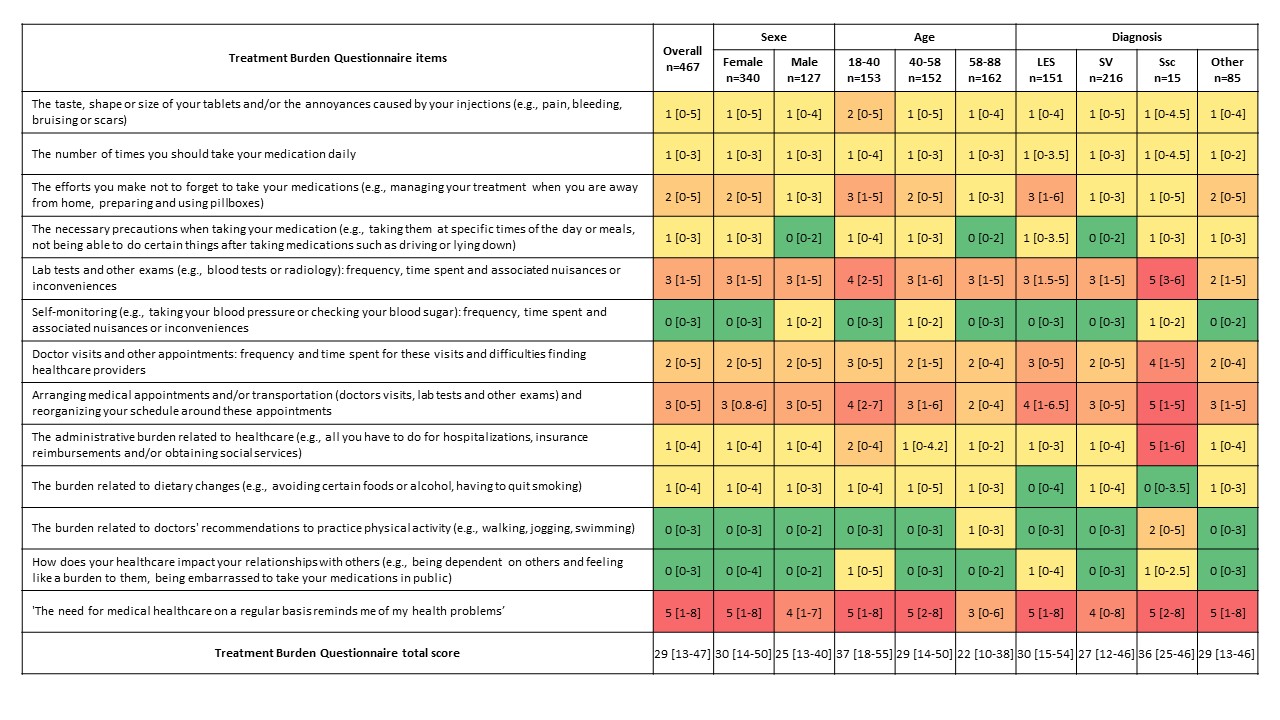 Figure 1. Scores for Treatment Burden Questionnaire.
Figure 1. Scores for Treatment Burden Questionnaire.
Footnote: Item scores are presented as median (IQR). Highest scores are in red and lowest in green.
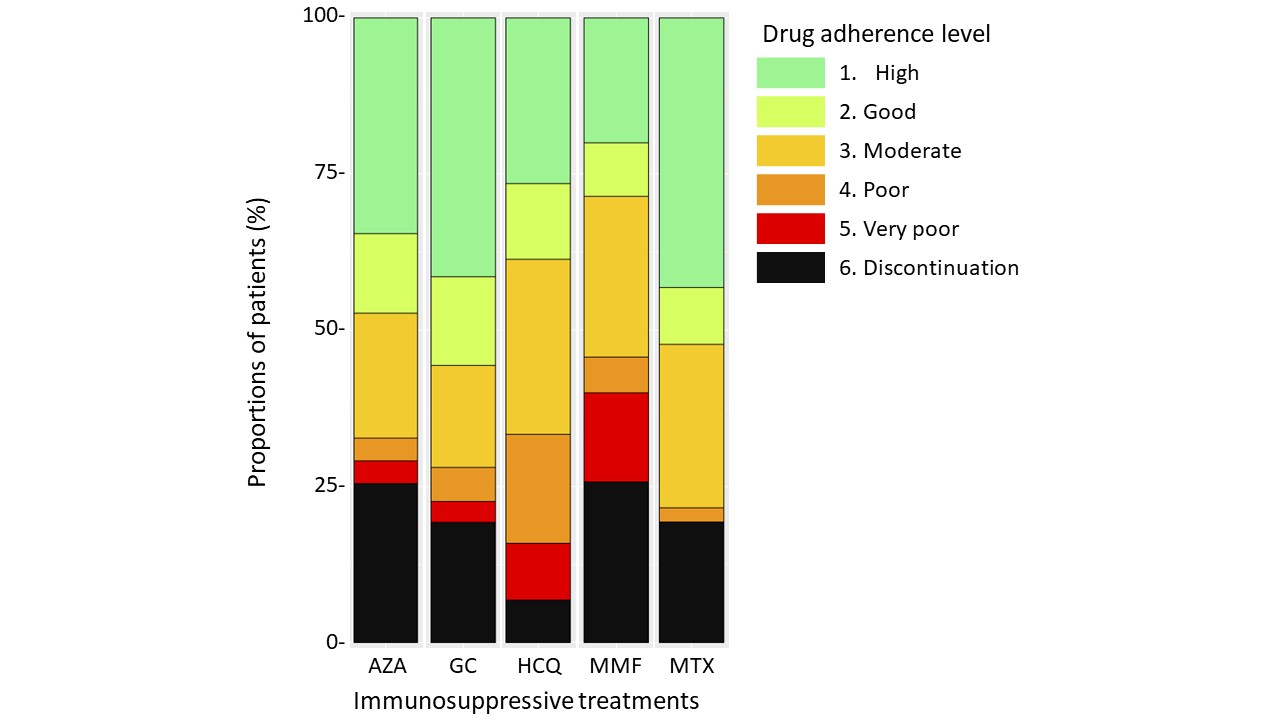 Figure 2. Drug adherence by different medication classes.
Figure 2. Drug adherence by different medication classes.
Footnote: Each bar corresponds to a medication class. Colours correspond to drug adherence levels.
Abbreviations: AZA, azathioprine; GC, glucocorticoids, HCQ, hydroxychloroquine; MMF, mycophenolate mofetil; MTX, methotrexate.
Disclosures: C. Mettler, None; V. Le Guern, None; P. Legendre, None; C. Morbieu, None; T. Swebel, None; X. Puéchal, Roche; P. Cohen, Roche; H. Kardaoui, None; A. Moores, None; L. Mouthon, Boehringer-Ingelheim, LFB; N. Costedoat-Chalumeau, UCB, Roche; B. Terrier, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb(BMS), Eli Lilly, LFB, Boehinger Ingelheim, Vifor Pharma, Pfizer, Roche.
Background/Purpose: Systemic autoimmune diseases (AID) are chronic conditions with significant burden related to the disease itself, its management, and treatments. Burden refers to the workload imposed by disease and healthcare on patients. Furthermore, management of these diseases may be impacted by non-adherence to treatment which can be influenced by many determinants. We aimed to describe factors associated with disease burden and medication adherence in patients with systemic AID and to assess the relationship between these two aspects.
Methods: We performed an observational study using an anonymous questionnaire among patients followed-up in our outpatient AID reference center. Disease burden was estimated by a validated score composed of 13 items, ranging from 0 (no burden) to 10 (maximum burden) (1,2). High burden was defined as greater than its median. For each treatment, medication adherence was assessed using a validated questionnaire to classify adherence into 6 levels (1: high level of adherence; 6: no treatment at all) (3). Logistic regression models and Spearman correlation test were performed.
Results: We included 467 patients, 73% were women and median age was 47 years (IQR 35.5-63). Main AID were systemic vasculitis in 46% and systemic lupus erythematosus, Sjögren's syndrome and/or antiphospholipid syndrome in 32%. Median disease duration was 7 years (IQR 3-13). Main treatments used were glucocorticoids (GCs) and hydroxychloroquine (HCQ) in 54% and 30% of patients, respectively. Median treatment burden score was 29/130 (IQR 13-47) (Figure 1). In univariate analysis, factors associated with high burden were age < 40 years, high number of physicians involved in disease management, number of drugs per day, number of daily tablets, active disease, GCs, azathioprine, and mycophenolate mofetil (MMF) use, and antidepressant treatment. In multivariate analysis, determinants that remained independently associated with high burden were age < 40 years (OR 2.25; 95% CI [1.27-4.03]), active disease (OR 4.24; 95% CI [2.53-7.25]), and GCs use (OR 2.21; 95% CI [1.38-3.55]). Median adherence to GCs and methotrexate were good (2/6), whereas adherence to azathioprine, HCQ and MMF were moderate (3/6) (Figure 2). GCs and azathioprine adherence scores were correlated with the importance attributed to treatments (p=0.014 and p< 0.001, respectively), whereas this association was not found for other treatments. Interestingly, disease burden and medication adherence scores were not significantly correlated in our cohort
Conclusion: High disease burden was significantly associated with younger age, active disease and GCs use, suggesting the need for special attention in these patients. Medication adherence was not always correlated with the importance attributed to it, nor with treatment burden. Studies evaluating the impact of specific actions would be of interest to decrease patient burden and to improve medication adherence.
 Figure 1. Scores for Treatment Burden Questionnaire.
Figure 1. Scores for Treatment Burden Questionnaire. Footnote: Item scores are presented as median (IQR). Highest scores are in red and lowest in green.
 Figure 2. Drug adherence by different medication classes.
Figure 2. Drug adherence by different medication classes.Footnote: Each bar corresponds to a medication class. Colours correspond to drug adherence levels.
Abbreviations: AZA, azathioprine; GC, glucocorticoids, HCQ, hydroxychloroquine; MMF, mycophenolate mofetil; MTX, methotrexate.
Disclosures: C. Mettler, None; V. Le Guern, None; P. Legendre, None; C. Morbieu, None; T. Swebel, None; X. Puéchal, Roche; P. Cohen, Roche; H. Kardaoui, None; A. Moores, None; L. Mouthon, Boehringer-Ingelheim, LFB; N. Costedoat-Chalumeau, UCB, Roche; B. Terrier, AstraZeneca, GlaxoSmithKline, Bristol-Myers Squibb(BMS), Eli Lilly, LFB, Boehinger Ingelheim, Vifor Pharma, Pfizer, Roche.

