Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0372–0402) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster I
0393: Are We Meeting Patients' Treatment Goals with Guideline-Based Therapy for Psoriatic Arthritis?
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- CS
Christeen Samuel, BA
Johns Hopkins University School of Medicine
Staten Island, NY, United States
Abstract Poster Presenter(s)
Christeen Samuel1, Laura Prichett1, Thomas Grader-Beck1, Uzma Haque1, John Miller1, Suzanne Grieb2 and Ana-Maria Orbai3, 1Johns Hopkins University School of Medicine, Baltimore, MD, 2Johns Hopkins University School of Medicine and School of Public Health, Baltimore, MD, 3Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD
Background/Purpose: Treatment success from the patients' perspective has not been defined in psoriatic arthritis (PsA). The objective was to determine prevalence and associations of patient-reported treatment success in PsA.
Methods: Consecutive patients with rheumatologist-diagnosed PsA, meeting the CASPAR classification, were recruited from a single center. The intervention was PsA guideline-based standard of care, which is defined as a review of disease activity and medication safety every 3-6 months and adjustment of therapy to meet treatment targets of low disease activity or remission (1-3). PsA outcome measures collected included: 68/66 tender/swollen joint counts, Leeds/SPARCC enthesitis indices, Leeds dactylitis index, psoriasis body surface area, patient reported outcomes (patient global, pain, PROMIS-29 Profile and additional short forms) and the patient acceptable treatment state (PASS). Treatment targets using DAPSA and MDA were calculated. The study primary outcome was a single-item question that assessed the patient's impression of treatment success: "Today, considering the level of control of your psoriatic arthritis and psoriasis, do you consider your treatment has been successful?" with response options Yes/No. A "No" choice branched to a drop-down list of reasons patients could select. Treatment success groups were compared using Student-t, Wilcoxon and Fisher's exact tests.
Results: A total of 106 participants had a baseline visit. Mean(SD) CASPAR score was 3.9 (1.0), mean age 51(13) years, mean BMI 31(7), 51% women, and 87% Caucasian. Seventy-two (68%) reported treatment success, and versus not, had significantly lower tender/swollen joint counts, dactylitis, psoriasis, and were more likely at treatment target by DAPSA and MDA; they also had better quality of life according to PROMIS-29 domains (Table 1a and b). Demographics and BMI were not associated with patient-reported treatment success. The most common reasons patients chose for treatment failure were pain (24%), psoriasis (15%), fatigue (12%) and decreased ability to be physically active (9%) (Table 3).
Conclusion: Patient-reported treatment success in PsA was associated with control of arthritis, dactylitis, psoriasis, and health-related quality of life. The top three patient-reported reasons for treatment failure were pain, psoriasis and fatigue.
References 1. Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol 2019;71:5–32.
2. Gossec L, Baraliakos X, Kerschbaumer A, Wit M de, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–712.
3. Coates LC, FitzGerald O, Merola JF, Smolen J, Mens LJJ van, Bertheussen H, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis/Outcome Measures in Rheumatology Consensus-Based Recommendations and Research Agenda for Use of Composite Measures and Treatment Targets in Psoriatic Arthritis. Arthritis Rheumatol 2018;70:345–355.
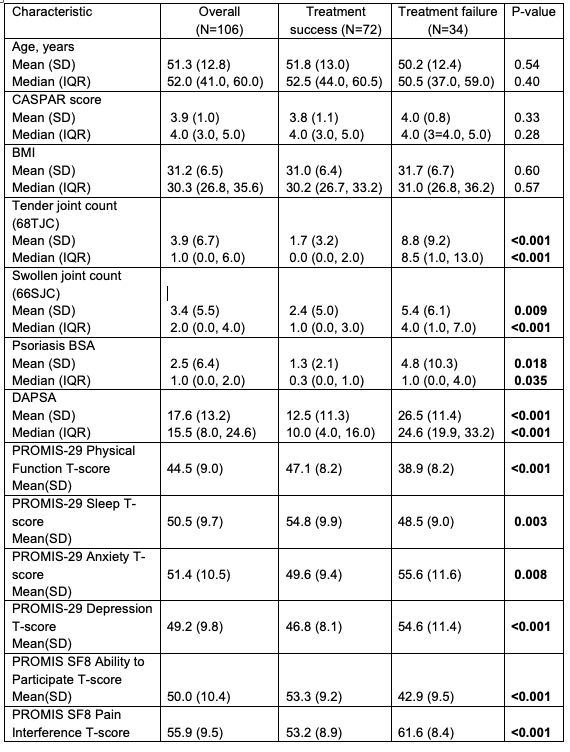 Table 1. Patient and disease characteristics (continuous variables)
Table 1. Patient and disease characteristics (continuous variables)
.jpg) Table 2. Patient and disease characteristics (count variables)
Table 2. Patient and disease characteristics (count variables)
.jpg) Table 3. Reasons for therapy failure
Table 3. Reasons for therapy failure
Disclosures: C. Samuel, None; L. Prichett, None; T. Grader-Beck, None; U. Haque, None; J. Miller, None; S. Grieb, None; A. Orbai, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Janssen, UCB.
Background/Purpose: Treatment success from the patients' perspective has not been defined in psoriatic arthritis (PsA). The objective was to determine prevalence and associations of patient-reported treatment success in PsA.
Methods: Consecutive patients with rheumatologist-diagnosed PsA, meeting the CASPAR classification, were recruited from a single center. The intervention was PsA guideline-based standard of care, which is defined as a review of disease activity and medication safety every 3-6 months and adjustment of therapy to meet treatment targets of low disease activity or remission (1-3). PsA outcome measures collected included: 68/66 tender/swollen joint counts, Leeds/SPARCC enthesitis indices, Leeds dactylitis index, psoriasis body surface area, patient reported outcomes (patient global, pain, PROMIS-29 Profile and additional short forms) and the patient acceptable treatment state (PASS). Treatment targets using DAPSA and MDA were calculated. The study primary outcome was a single-item question that assessed the patient's impression of treatment success: "Today, considering the level of control of your psoriatic arthritis and psoriasis, do you consider your treatment has been successful?" with response options Yes/No. A "No" choice branched to a drop-down list of reasons patients could select. Treatment success groups were compared using Student-t, Wilcoxon and Fisher's exact tests.
Results: A total of 106 participants had a baseline visit. Mean(SD) CASPAR score was 3.9 (1.0), mean age 51(13) years, mean BMI 31(7), 51% women, and 87% Caucasian. Seventy-two (68%) reported treatment success, and versus not, had significantly lower tender/swollen joint counts, dactylitis, psoriasis, and were more likely at treatment target by DAPSA and MDA; they also had better quality of life according to PROMIS-29 domains (Table 1a and b). Demographics and BMI were not associated with patient-reported treatment success. The most common reasons patients chose for treatment failure were pain (24%), psoriasis (15%), fatigue (12%) and decreased ability to be physically active (9%) (Table 3).
Conclusion: Patient-reported treatment success in PsA was associated with control of arthritis, dactylitis, psoriasis, and health-related quality of life. The top three patient-reported reasons for treatment failure were pain, psoriasis and fatigue.
References 1. Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation Guideline for the Treatment of Psoriatic Arthritis. Arthritis Rheumatol 2019;71:5–32.
2. Gossec L, Baraliakos X, Kerschbaumer A, Wit M de, McInnes I, Dougados M, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis 2020;79:700–712.
3. Coates LC, FitzGerald O, Merola JF, Smolen J, Mens LJJ van, Bertheussen H, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis/Outcome Measures in Rheumatology Consensus-Based Recommendations and Research Agenda for Use of Composite Measures and Treatment Targets in Psoriatic Arthritis. Arthritis Rheumatol 2018;70:345–355.
 Table 1. Patient and disease characteristics (continuous variables)
Table 1. Patient and disease characteristics (continuous variables).jpg) Table 2. Patient and disease characteristics (count variables)
Table 2. Patient and disease characteristics (count variables).jpg) Table 3. Reasons for therapy failure
Table 3. Reasons for therapy failureDisclosures: C. Samuel, None; L. Prichett, None; T. Grader-Beck, None; U. Haque, None; J. Miller, None; S. Grieb, None; A. Orbai, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Janssen, UCB.

