Back
Poster Session C
Vasculitis
Session: (1543–1578) Vasculitis – Non-ANCA-Associated and Related Disorders Poster II
1552: Rare Variant Analysis of Aortopathy Genes in Takayasu’s Arteritis
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- HA
Hugh Alessi, BA
University of Washington School of Medicine & The National Institute of Arthritis and Musculoskeletal and Skin Diseases
Moscow, ID, United States
Abstract Poster Presenter(s)
Hugh Alessi1, Yiming Luo1, Kaitlin Quinn2 and Peter Grayson3, 1NIH, Bethesda, MD, 2National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH), Bethesda, MD, 3National Institutes of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), National Institutes of Health (NIH), Bethesda, MD
Background/Purpose: A set of genes has been associated with aortopathies, which are defined as non-inflammatory diseases where the integrity of large arteries is compromised leading to dilatation, aneurysm, and dissections. Overlap can exist between the clinical features of aortopathies and Takayasu's arteritis (TAK), a form of large vessel vasculitis characterized by vascular inflammation of the aorta and branch vessels. Genetic aortopathy panels are commercially available; however, the clinical utility of such screening in patients with TAK is unclear. We hypothesized (1) that genes implicated in aortopathies could be involved in the pathogenesis of TAK and (2) that rare variants in specific genes may be associated with clinical features in TAK.
Methods: Patients with TAK and healthy controls were sequenced via whole exome sequencing. A list of aortopathy genes was chosen based upon their inclusion in one of 10 commercial aortopathy panels. Genetic sequencing data and identified rare variants were subject to quality control steps ensuring sufficient read depth and coverage harmonization between patients and controls. Rare variants in these genes were then evaluated in two steps. First, a series of gene-level burden tests comprising 16 models of genetic variation was used to compare the burden of rare variants in TAK versus healthy controls. Second, patients were evaluated for the presence of a clinical feature associated with one of six phenotypic groupings: aneurysm, occlusion, surgery, myocardial infarction, stroke, and thromboembolism. The burden of rare variants in aortopathy genes was then examined in TAK patients with and without a qualifying clinical feature. Firth's Logistic Regression, Fisher's Exact test, and Optimal Sequence Kernel Association Test (SKAT-O) were used for burden testing. A significance cut-off for gene-level burden tests was set at 5.0 x 10-5, and significance for phenotype associations was set at p < 0.0083 using Bonferroni correction.
Results: Rare variants were examined in 62 aortopathy genes in 153 patients with TAK and 2923 healthy controls. No genes were found to be significantly associated with TAK (FIGURE 1). For the phenotype association analysis, rare variants in COL4A5 were significantly associated with surgical intervention (arterial graft or valve replacement) in TAK (p = 0.0007). Variants in ADAMTS2 were significantly associated with stroke in TAK (p = 0.008) (FIGURE 2).
Conclusion: While rare variants in genes associated with aortopathies are present in TAK, there is not a significantly greater burden of rare variants in these genes in patients with TAK compared to healthy controls. Commercial aortopathy gene panel testing thus does not appear to yield any benefit to the clinical management of patients with TAK. Many variants with uncertain significance were identified, and two gene-phenotypic groupings were discovered. Continued research into potential functional roles of these genes in TAK is warranted. Future studies with larger numbers of patients may be better powered to identify clinically meaningful rare variant associations in TAK.
.jpg) Figure 1. Burden test results for 62 aortopathy genes across 16 statistical models
Figure 1. Burden test results for 62 aortopathy genes across 16 statistical models
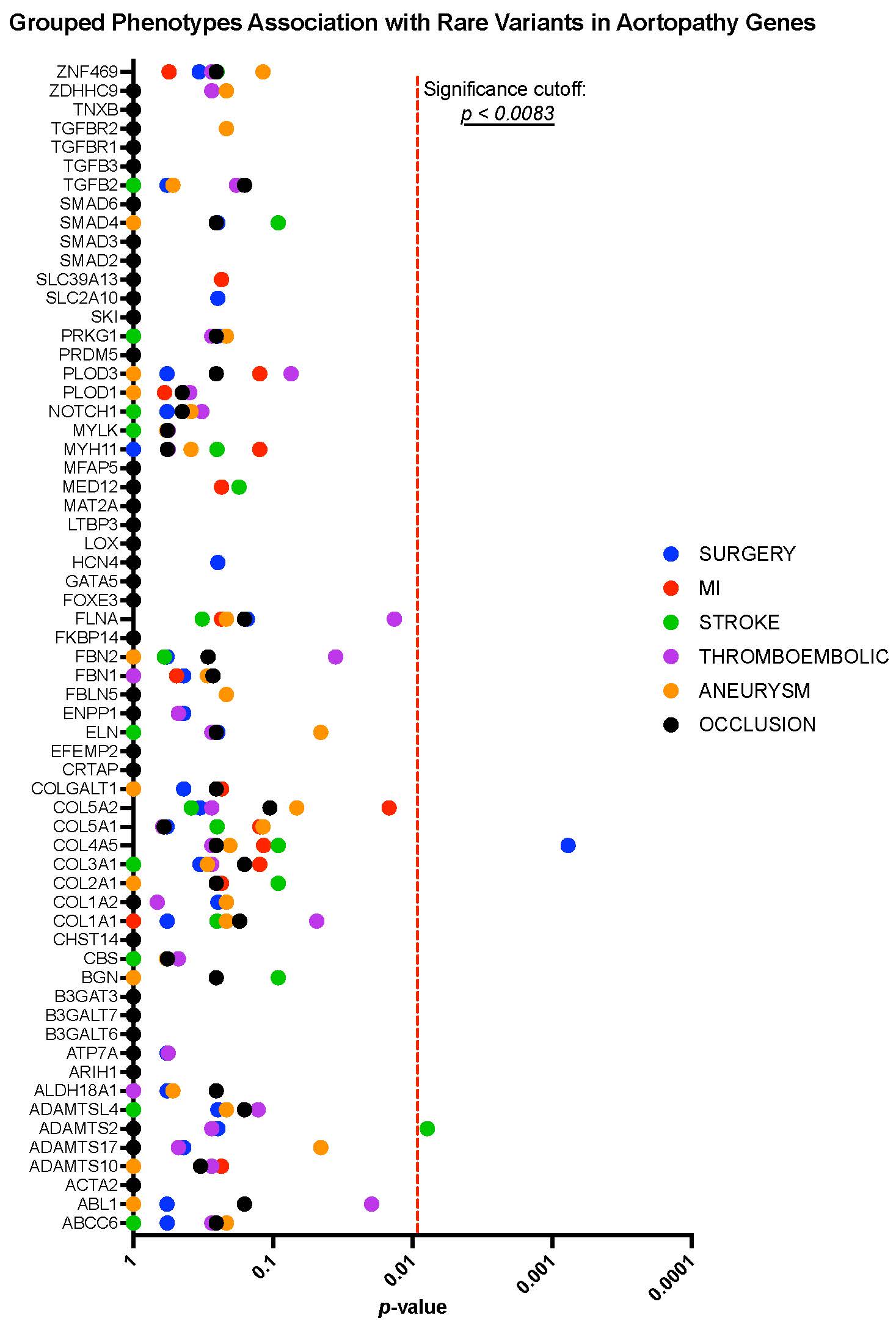 Figure 2. Phenotyopic associations with rare variants in aortopathy genes
Figure 2. Phenotyopic associations with rare variants in aortopathy genes
Disclosures: H. Alessi, None; Y. Luo, None; K. Quinn, None; P. Grayson, None.
Background/Purpose: A set of genes has been associated with aortopathies, which are defined as non-inflammatory diseases where the integrity of large arteries is compromised leading to dilatation, aneurysm, and dissections. Overlap can exist between the clinical features of aortopathies and Takayasu's arteritis (TAK), a form of large vessel vasculitis characterized by vascular inflammation of the aorta and branch vessels. Genetic aortopathy panels are commercially available; however, the clinical utility of such screening in patients with TAK is unclear. We hypothesized (1) that genes implicated in aortopathies could be involved in the pathogenesis of TAK and (2) that rare variants in specific genes may be associated with clinical features in TAK.
Methods: Patients with TAK and healthy controls were sequenced via whole exome sequencing. A list of aortopathy genes was chosen based upon their inclusion in one of 10 commercial aortopathy panels. Genetic sequencing data and identified rare variants were subject to quality control steps ensuring sufficient read depth and coverage harmonization between patients and controls. Rare variants in these genes were then evaluated in two steps. First, a series of gene-level burden tests comprising 16 models of genetic variation was used to compare the burden of rare variants in TAK versus healthy controls. Second, patients were evaluated for the presence of a clinical feature associated with one of six phenotypic groupings: aneurysm, occlusion, surgery, myocardial infarction, stroke, and thromboembolism. The burden of rare variants in aortopathy genes was then examined in TAK patients with and without a qualifying clinical feature. Firth's Logistic Regression, Fisher's Exact test, and Optimal Sequence Kernel Association Test (SKAT-O) were used for burden testing. A significance cut-off for gene-level burden tests was set at 5.0 x 10-5, and significance for phenotype associations was set at p < 0.0083 using Bonferroni correction.
Results: Rare variants were examined in 62 aortopathy genes in 153 patients with TAK and 2923 healthy controls. No genes were found to be significantly associated with TAK (FIGURE 1). For the phenotype association analysis, rare variants in COL4A5 were significantly associated with surgical intervention (arterial graft or valve replacement) in TAK (p = 0.0007). Variants in ADAMTS2 were significantly associated with stroke in TAK (p = 0.008) (FIGURE 2).
Conclusion: While rare variants in genes associated with aortopathies are present in TAK, there is not a significantly greater burden of rare variants in these genes in patients with TAK compared to healthy controls. Commercial aortopathy gene panel testing thus does not appear to yield any benefit to the clinical management of patients with TAK. Many variants with uncertain significance were identified, and two gene-phenotypic groupings were discovered. Continued research into potential functional roles of these genes in TAK is warranted. Future studies with larger numbers of patients may be better powered to identify clinically meaningful rare variant associations in TAK.
.jpg) Figure 1. Burden test results for 62 aortopathy genes across 16 statistical models
Figure 1. Burden test results for 62 aortopathy genes across 16 statistical models Figure 2. Phenotyopic associations with rare variants in aortopathy genes
Figure 2. Phenotyopic associations with rare variants in aortopathy genesDisclosures: H. Alessi, None; Y. Luo, None; K. Quinn, None; P. Grayson, None.

