Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0287: Association Between Short-Term Response to Upadacitinib Treatment and Long-Term Clinical Outcomes in Patients with Rheumatoid Arthritis and Prior Inadequate Response to Tumor Necrosis Factor Inhibitor Therapy
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- CC
Christina Charles-Schoeman, MD, MS
UCLA Medical Center
Santa Monica, CA, United States
Abstract Poster Presenter(s)
Christina Charles-Schoeman1, Roy Fleischmann2, Stephen Hall3, Arthur Kavanaugh4, Andrea Rubbert-Roth5, Ryan DeMasi6, Sara Penn6, Andrew Garrison6, Samuel Anyanwu6, Radames Sierra-Zorita7 and Ricardo Xavier8, 1Division of Rheumatology, University of California, Los Angeles, Santa Monica, CA, 2University of Texas Southwestern Medical Center and Metroplex Clinical Research Center, Dallas, TX, 3Emeritus Research and Monash University, Melbourne, Australia, 4University of California San Diego, La Jolla, CA, 5Division of Rheumatology, Cantonal Clinic St Gallen, St.Gallen, Switzerland, 6AbbVie, Inc., North Chicago, IL, 7University of Puerto Rico, San Juan, Puerto Rico, 8Hospital de Clínicas de Porto Alegre, Porto Alegre, Brazil
Background/Purpose: Early predictors of response to treatment with upadacitinib (UPA), an oral Janus kinase inhibitor, could help to optimize a treat-to-target approach in patients with RA. This post hoc analysis evaluated whether early response to UPA treatment correlated with subsequent achievement of long-term clinical outcomes in patients with RA in whom ≥1 TNF inhibitor had previously failed.
Methods: Data on patients who received UPA 15 mg once daily in whom ≥1 TNF inhibitor had failed (including patients with inadequate response or intolerance; TNFi-IR) were analyzed from two Phase 3 trials: SELECT-BEYOND and SELECT-CHOICE. Positive predictive value (PPV) and negative predictive value (NPV) were used to assess whether a ≥6-point improvement in Clinical Disease Activity Index (CDAI) and a ≥1.2-point improvement in DAS 28-joint count with CRP (DAS28-CRP) at Weeks 4 and 12 predicted achievement of CDAI low disease activity (LDA) or remission (≤10 or ≤2.8, respectively) and DAS28-CRP ≤3.2 and < 2.6, at Weeks 24 and 48 in SELECT-BEYOND, and at Week 24 in SELECT-CHOICE. Non-responder imputation was used for missing data.
Results: The analysis included 140 TNFi-IR patients (89% inadequate response, 11% intolerant) from SELECT-BEYOND and 258 TNFi-IR patients (84% inadequate response, 16% intolerant) from SELECT-CHOICE. Across the studies, CDAI LDA and remission were achieved by 50–58% and 11–23% of patients, respectively (Figure 1). At Weeks 4 and 12 in SELECT-BEYOND, 31 (22%) and 14 (10%) patients, respectively, failed to achieve CDAI improvement ≥6 from baseline (Table 1). Of these, 9 (29%) and 2 (14%) patients achieved LDA, and 1 (3%) and 0 achieved remission at Week 24. The Week 24 NPVs for CDAI LDA were higher for Week 12 (86%) than Week 4 (71%); but similar for remission (97% [Week 4] and 100% [Week 12]). At Week 48, 12 patients (39%) and 1 patient (7%) without ≥6 CDAI improvement at Weeks 4 and 12, respectively, achieved CDAI LDA, while 2 (6%) and 0 achieved remission. The patterns and magnitude of the corresponding Week 48 NPVs were similar to those at Week 24 (Table 1). In SELECT-CHOICE, similar patterns to SELECT-BEYOND were seen at Week 24 in patients who did achieve CDAI improvement ≥6 from baseline (Table 2). Similar trends were observed when assessing the ability of DAS28 CRP improvement < 1.2/≥1.2 from baseline to Weeks 4 and 12 to predict DAS28 CRP ≤3.2 and < 2.6 treatment targets at Weeks 24 and 48 in SELECT-BEYOND and at Week 24 in SELECT-CHOICE.
Conclusion: Although most patients receiving UPA achieved early improvements in CDAI and DAS28-CRP scores, patients who did not demonstrate improvements of ≥6 in CDAI or ≥1.2 in DAS28-CRP within 12 weeks were unlikely to achieve disease activity targets with long-term treatment. Therefore, these patients may benefit from therapy adjustment, consistent with treat-to-target recommendations.
.jpg) Table 1. Probabilities of achieving long-term CDAI treatment targets in SELECT-BEYOND based on Week 4 and 12 responses
Table 1. Probabilities of achieving long-term CDAI treatment targets in SELECT-BEYOND based on Week 4 and 12 responses
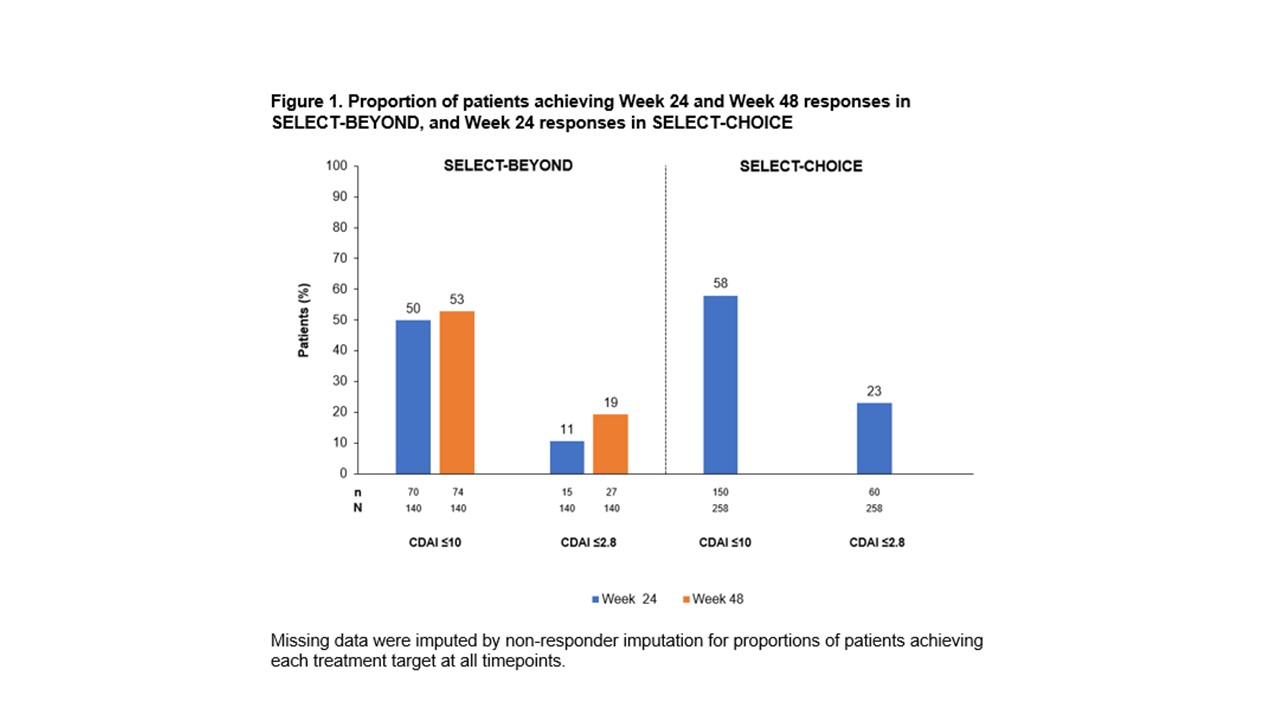 Figure 1. Proportion of patients achieving Week 24 and Week 48 responses in SELECT BEYOND, and Week 24 responses in SELECT CHOICE
Figure 1. Proportion of patients achieving Week 24 and Week 48 responses in SELECT BEYOND, and Week 24 responses in SELECT CHOICE
Disclosures: C. Charles-Schoeman, AbbVie, Bristol Myers Squibb (BMS), Pfizer, Regeneron-Sanofi, Gilead; R. Fleischmann, AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galvani, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, UCB, Galapagos; S. Hall, AbbVie, Bristol-Myers Squibb (BMS), Lilly, Janssen, UCB, Novartis, Amgen, Gilead, Merck; A. Kavanaugh, AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb(BMS), Celgene, Centocor-Janssen, Pfizer, Roche, UCB; A. Rubbert-Roth, None; R. DeMasi, AbbVie; S. Penn, AbbVie; A. Garrison, AbbVie Inc.; S. Anyanwu, AbbVie; R. Sierra-Zorita, AbbVie; R. Xavier, AbbVie, Lilly, Janssen, Novartis, Pfizer, Roche.
Background/Purpose: Early predictors of response to treatment with upadacitinib (UPA), an oral Janus kinase inhibitor, could help to optimize a treat-to-target approach in patients with RA. This post hoc analysis evaluated whether early response to UPA treatment correlated with subsequent achievement of long-term clinical outcomes in patients with RA in whom ≥1 TNF inhibitor had previously failed.
Methods: Data on patients who received UPA 15 mg once daily in whom ≥1 TNF inhibitor had failed (including patients with inadequate response or intolerance; TNFi-IR) were analyzed from two Phase 3 trials: SELECT-BEYOND and SELECT-CHOICE. Positive predictive value (PPV) and negative predictive value (NPV) were used to assess whether a ≥6-point improvement in Clinical Disease Activity Index (CDAI) and a ≥1.2-point improvement in DAS 28-joint count with CRP (DAS28-CRP) at Weeks 4 and 12 predicted achievement of CDAI low disease activity (LDA) or remission (≤10 or ≤2.8, respectively) and DAS28-CRP ≤3.2 and < 2.6, at Weeks 24 and 48 in SELECT-BEYOND, and at Week 24 in SELECT-CHOICE. Non-responder imputation was used for missing data.
Results: The analysis included 140 TNFi-IR patients (89% inadequate response, 11% intolerant) from SELECT-BEYOND and 258 TNFi-IR patients (84% inadequate response, 16% intolerant) from SELECT-CHOICE. Across the studies, CDAI LDA and remission were achieved by 50–58% and 11–23% of patients, respectively (Figure 1). At Weeks 4 and 12 in SELECT-BEYOND, 31 (22%) and 14 (10%) patients, respectively, failed to achieve CDAI improvement ≥6 from baseline (Table 1). Of these, 9 (29%) and 2 (14%) patients achieved LDA, and 1 (3%) and 0 achieved remission at Week 24. The Week 24 NPVs for CDAI LDA were higher for Week 12 (86%) than Week 4 (71%); but similar for remission (97% [Week 4] and 100% [Week 12]). At Week 48, 12 patients (39%) and 1 patient (7%) without ≥6 CDAI improvement at Weeks 4 and 12, respectively, achieved CDAI LDA, while 2 (6%) and 0 achieved remission. The patterns and magnitude of the corresponding Week 48 NPVs were similar to those at Week 24 (Table 1). In SELECT-CHOICE, similar patterns to SELECT-BEYOND were seen at Week 24 in patients who did achieve CDAI improvement ≥6 from baseline (Table 2). Similar trends were observed when assessing the ability of DAS28 CRP improvement < 1.2/≥1.2 from baseline to Weeks 4 and 12 to predict DAS28 CRP ≤3.2 and < 2.6 treatment targets at Weeks 24 and 48 in SELECT-BEYOND and at Week 24 in SELECT-CHOICE.
Conclusion: Although most patients receiving UPA achieved early improvements in CDAI and DAS28-CRP scores, patients who did not demonstrate improvements of ≥6 in CDAI or ≥1.2 in DAS28-CRP within 12 weeks were unlikely to achieve disease activity targets with long-term treatment. Therefore, these patients may benefit from therapy adjustment, consistent with treat-to-target recommendations.
.jpg) Table 1. Probabilities of achieving long-term CDAI treatment targets in SELECT-BEYOND based on Week 4 and 12 responses
Table 1. Probabilities of achieving long-term CDAI treatment targets in SELECT-BEYOND based on Week 4 and 12 responses Figure 1. Proportion of patients achieving Week 24 and Week 48 responses in SELECT BEYOND, and Week 24 responses in SELECT CHOICE
Figure 1. Proportion of patients achieving Week 24 and Week 48 responses in SELECT BEYOND, and Week 24 responses in SELECT CHOICEDisclosures: C. Charles-Schoeman, AbbVie, Bristol Myers Squibb (BMS), Pfizer, Regeneron-Sanofi, Gilead; R. Fleischmann, AbbVie, Amgen, Bristol Myers Squibb, Eli Lilly, Galvani, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, UCB, Galapagos; S. Hall, AbbVie, Bristol-Myers Squibb (BMS), Lilly, Janssen, UCB, Novartis, Amgen, Gilead, Merck; A. Kavanaugh, AbbVie, Amgen, AstraZeneca, Bristol-Myers Squibb(BMS), Celgene, Centocor-Janssen, Pfizer, Roche, UCB; A. Rubbert-Roth, None; R. DeMasi, AbbVie; S. Penn, AbbVie; A. Garrison, AbbVie Inc.; S. Anyanwu, AbbVie; R. Sierra-Zorita, AbbVie; R. Xavier, AbbVie, Lilly, Janssen, Novartis, Pfizer, Roche.

