Back
Poster Session C
Rheumatoid arthritis (RA)
Session: (1387–1416) RA – Diagnosis, Manifestations, and Outcomes Poster III
1387: Real-World Utilization of Adalimumab Biosimilar (ABP 501) in Patients with Rheumatoid Arthritis, Ankylosing Spondylitis and Psoriatic Arthritis in Europe
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- RJ
Ran Jin, PhD
Amgen Inc.
Thousand Oaks, CA, United States
Abstract Poster Presenter(s)
Ran Jin1, Megan Hughes2, Emily Goddard2, James Piercy2, Delphine Courmier2, Rachael Meadows2 and Waldemar Radziszewski1, 1Amgen, Inc., Thousand Oaks, CA, 2Adelphi Group, Bollington, United Kingdom
Background/Purpose: ABP 501 is the first adalimumab biosimilar approved by the European Medicines Agency. A clinical trial demonstrated biosimilarity between ABP 501 and the reference product (RP) in terms of efficacy, safety, and immunogenicity in adalimumab-naïve adult patients with moderate to severe rheumatoid arthritis (RA). We aimed to evaluate satisfaction of patients currently treated with ABP 501 and assess reasons for switch from RP in the real-world setting for RA and indications approved on the basis of extrapolation [ankylosing spondylitis (AS), psoriatic arthritis (PsA)].
Methods: Data were collected from the RA, AS, and PsA 2021 Adelphi Disease Specific Programmes (DSP™), a point-in-time survey of physicians and their consulting patients conducted in France, Germany, Italy, Spain, and the United Kingdom. Rheumatologists and dermatologists (PsA only) completed surveys detailing patient medical record data. Patients voluntarily completed questionnaires regarding satisfaction with current treatment, Work Productivity and Activity Impairment (WPAI) questionnaire and the Euro-QoL 5-dimension 5-level quality of life (EQ5D-5L) survey. Descriptive analyses were conducted for 2 patient groups: adalimumab-naïve users of ABP 501 as first advanced therapy (AT) ("Initiators"), and patients who switched from RP (as first AT) to ABP 501 as current treatment ("Switchers"). All patients initiated ABP 501 after October 2018 (market availability) and were ≥18 years at data collection.
Results: A total of 1,025 patients across 3 indications (RA=413, AS=194, PsA=418) were included in the analysis, with majority being Initiators. Patient demographic characteristics were slightly different between Initiators and Switchers within disease cohorts (Table 1). Approximately 1/3 of all RA patients, almost all AS patients ( >97%) and most PsA patients ( >82%) were receiving ABP 501 as monotherapy (Table 1). Among Initiators, median duration of ABP 501 treatment was similar across indications (RA: 12.3 months, AS: 10.7 months, PsA: 10.4 months). A much greater proportion of Initiators had mild disease at time of reporting than at therapy initiation (RA, 77.1% vs. 8.6%; AS, 63.2% vs. 7.1%; PsA, 67.8% vs. 8.3%). Time on RP prior to switch varied by disease indication (Table 1) but median duration of ABP 501 following switch was similar (RA: 12.4 months, AS: 15.3 months, PsA: 14.1 months). Among Switchers, the proportion of patients with mild disease was larger at the time of reporting than at ABP 501 initiation (RA, 84.1% vs. 52.4%; AS, 75.0% vs. 32.5%; PsA, 81.5% vs. 33.3%). The patient reported outcome measures (PROMs) EQ5D-5L and WPAI were mostly similar at the time of reporting, regardless of indication and prior exposure to RP. Both physicians and patients reported a high level of treatment satisfaction ( >86%) across all groups (Table 1). Switch was mainly due to financial reasons or formulary driven switch (Figure 1).
Conclusion: Patient and physician satisfaction with ABP 501 in the real world was high for both Initiators and Switchers across all 3 indications. The most frequently reported reasons for switch from RP to ABP 501 were financial and formulary driven reasons.
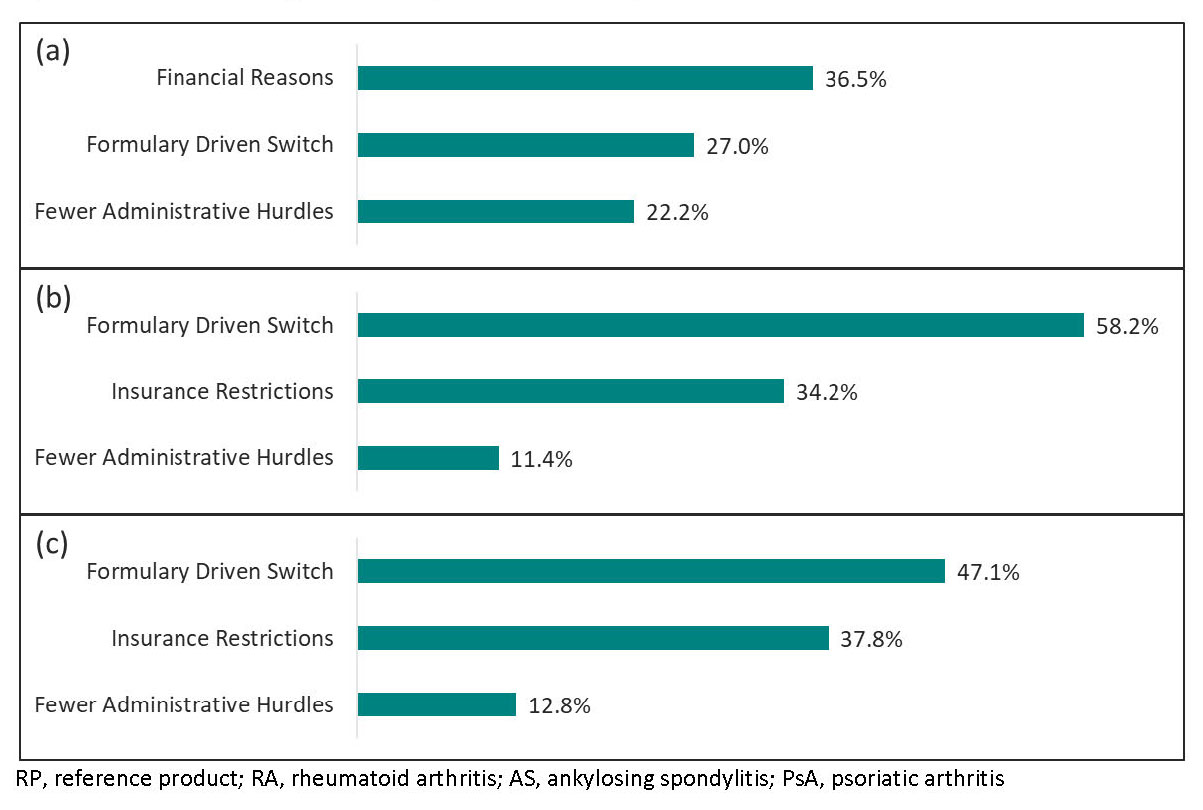 Figure 1: Top 3 reasons for switch from RP to ABP 501 reported by physicians for (a) RA Switchers (n=63), (b) AS Switchers (n=80), (c) PsA Switchers (n=173). Reported for switcher groups from multiple choice answer options that varied between indications and were not mutually exclusive.
Figure 1: Top 3 reasons for switch from RP to ABP 501 reported by physicians for (a) RA Switchers (n=63), (b) AS Switchers (n=80), (c) PsA Switchers (n=173). Reported for switcher groups from multiple choice answer options that varied between indications and were not mutually exclusive.
.jpg) Table 1: Demographics, clinical characteristics and PROMs for patients receiving ABP 501 across indications
Table 1: Demographics, clinical characteristics and PROMs for patients receiving ABP 501 across indications
Disclosures: R. Jin, Amgen; M. Hughes, None; E. Goddard, None; J. Piercy, Adelphi Real World; D. Courmier, None; R. Meadows, None; W. Radziszewski, Amgen.
Background/Purpose: ABP 501 is the first adalimumab biosimilar approved by the European Medicines Agency. A clinical trial demonstrated biosimilarity between ABP 501 and the reference product (RP) in terms of efficacy, safety, and immunogenicity in adalimumab-naïve adult patients with moderate to severe rheumatoid arthritis (RA). We aimed to evaluate satisfaction of patients currently treated with ABP 501 and assess reasons for switch from RP in the real-world setting for RA and indications approved on the basis of extrapolation [ankylosing spondylitis (AS), psoriatic arthritis (PsA)].
Methods: Data were collected from the RA, AS, and PsA 2021 Adelphi Disease Specific Programmes (DSP™), a point-in-time survey of physicians and their consulting patients conducted in France, Germany, Italy, Spain, and the United Kingdom. Rheumatologists and dermatologists (PsA only) completed surveys detailing patient medical record data. Patients voluntarily completed questionnaires regarding satisfaction with current treatment, Work Productivity and Activity Impairment (WPAI) questionnaire and the Euro-QoL 5-dimension 5-level quality of life (EQ5D-5L) survey. Descriptive analyses were conducted for 2 patient groups: adalimumab-naïve users of ABP 501 as first advanced therapy (AT) ("Initiators"), and patients who switched from RP (as first AT) to ABP 501 as current treatment ("Switchers"). All patients initiated ABP 501 after October 2018 (market availability) and were ≥18 years at data collection.
Results: A total of 1,025 patients across 3 indications (RA=413, AS=194, PsA=418) were included in the analysis, with majority being Initiators. Patient demographic characteristics were slightly different between Initiators and Switchers within disease cohorts (Table 1). Approximately 1/3 of all RA patients, almost all AS patients ( >97%) and most PsA patients ( >82%) were receiving ABP 501 as monotherapy (Table 1). Among Initiators, median duration of ABP 501 treatment was similar across indications (RA: 12.3 months, AS: 10.7 months, PsA: 10.4 months). A much greater proportion of Initiators had mild disease at time of reporting than at therapy initiation (RA, 77.1% vs. 8.6%; AS, 63.2% vs. 7.1%; PsA, 67.8% vs. 8.3%). Time on RP prior to switch varied by disease indication (Table 1) but median duration of ABP 501 following switch was similar (RA: 12.4 months, AS: 15.3 months, PsA: 14.1 months). Among Switchers, the proportion of patients with mild disease was larger at the time of reporting than at ABP 501 initiation (RA, 84.1% vs. 52.4%; AS, 75.0% vs. 32.5%; PsA, 81.5% vs. 33.3%). The patient reported outcome measures (PROMs) EQ5D-5L and WPAI were mostly similar at the time of reporting, regardless of indication and prior exposure to RP. Both physicians and patients reported a high level of treatment satisfaction ( >86%) across all groups (Table 1). Switch was mainly due to financial reasons or formulary driven switch (Figure 1).
Conclusion: Patient and physician satisfaction with ABP 501 in the real world was high for both Initiators and Switchers across all 3 indications. The most frequently reported reasons for switch from RP to ABP 501 were financial and formulary driven reasons.
 Figure 1: Top 3 reasons for switch from RP to ABP 501 reported by physicians for (a) RA Switchers (n=63), (b) AS Switchers (n=80), (c) PsA Switchers (n=173). Reported for switcher groups from multiple choice answer options that varied between indications and were not mutually exclusive.
Figure 1: Top 3 reasons for switch from RP to ABP 501 reported by physicians for (a) RA Switchers (n=63), (b) AS Switchers (n=80), (c) PsA Switchers (n=173). Reported for switcher groups from multiple choice answer options that varied between indications and were not mutually exclusive..jpg) Table 1: Demographics, clinical characteristics and PROMs for patients receiving ABP 501 across indications
Table 1: Demographics, clinical characteristics and PROMs for patients receiving ABP 501 across indicationsDisclosures: R. Jin, Amgen; M. Hughes, None; E. Goddard, None; J. Piercy, Adelphi Real World; D. Courmier, None; R. Meadows, None; W. Radziszewski, Amgen.

