Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0317–0342) SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
0333: Autoantibody Trajectories Associate with Classification and Treatment Response in Lupus Nephritis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Andrea Fava, MD
Johns Hopkins University
Baltimore, MD, United States
Abstract Poster Presenter(s)
Andrea Fava1, Carla J. Guthridge2, Joseph Kheir2, Catriona Wagner3, Michelle Petri4, Jill Buyon5, Betty Diamond6, the Accelerating Medicines Partnership (AMP) RA/SLE7, Joel Guthridge2 and Judith James2, 1Johns Hopkins University, Baltimore, MD, 2Oklahoma Medical Research Foundation, Oklahoma City, OK, 3Oklahoma Medical Research Foundation, Oklahoma, 4Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, 5NYU Grossman School of Medicine, New York, NY, 6Feinstein Institutes for Medical Research, Manhasset, NY, 7Multiple Insitutions
Background/Purpose: Autoantibodies are a hallmark of lupus nephritis (LN). While there is known heterogeneity in autoantibody expression among LN patients, the association of autoantibodies with LN subtypes and the implications of longitudinal changes in LN are not entirely understood. In this study, we quantified circulating autoantibodies in the Accelerating Medicines Partnership (AMP) LN longitudinal cohort to identify novel serum biomarkers of LN classification and treatment response and to provide insights into the pathogenesis of LN.
Methods: SLE patients meeting ACR or SLICC criteria (n=268) indicated for kidney biopsy by a urine protein/creatinine (UPCR) >0.5 were recruited for this study as part of the AMP. Kidney biopsies were evaluated by a renal pathologist according to ISN/RPS classification, and serum samples were collected at the time of diagnostic kidney biopsy and 3-, 6-, and 12-months post-biopsy. Serum autoantibodies against dsDNA, chromatin, ribosomal-P, Ro, La, Sm, SmRNP, RNP, Jo-1, Scl-70, and centromere-B were measured using the BioPlex 2200® ANA kit (Bio-Rad Technologies, Hercules, CA), while anti-C1q positivity was determined by ELISA (QUANTA Lite®, Werfen, Bedford, MA). Clinical response was determined at 12 months using the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study definitions for patients with ISN/RPS class III, IV, V, or a combination thereof and a baseline UPCR ratio >1.0.
Results: Most LN patients exhibited autoantibodies against chromatin (78%) dsDNA (70%), SmRNP (63%), C1q (56%), RNP (54%), and Sm (52%) (Figure 1A). Patients with proliferative LN (class III, IV, III+V, or IV+V) had higher positivity rates of several autoantibodies, including those against dsDNA, chromatin, and C1q, compared to patients with membranous LN (class V) (Figure 1A). Similarly, patients with pure proliferative (class III or IV) and mixed (class III+V or IV+V) LN had significantly higher titers of these autoantibodies compared to those with mesangial (class I or II), membranous, and advanced sclerosis (class VI) LN (Figure 1B-D). Furthermore, increased titers of these autoantibodies were associated with higher odds of having proliferative LN (Table). Proliferative LN patients with a complete treatment response exhibited a significant decline in several autoantibodies including anti-dsDNA, C1q, chromatin, Smith, and ribosomal P (Figure 2). Autoantibody levels remained relatively stable in partial- and non-responder proliferative LN patients, as well as in patients with membranous LN.
Conclusion: LN patients exhibit heterogeneous autoantibody profiles associated with ISN/RPS classification. Specifically, levels of autoantibodies against dsDNA, C1q, chromatin, and ribosomal P may serve as noninvasive biomarkers of proliferative LN. In patients with proliferative but not membranous LN, a decline in the titers of several autoantibodies, including many not routinely measured over time, such as anti-Sm, was associated with treatment response, suggesting a possible role in LN pathogenesis. In addition, these autoantibodies may serve as early biomarkers of treatment response.
.jpg) Figure 1. Prevalence and concentrations of autoantibodies at lupus nephritis (LN) diagnosis according to LN classification. (A) Overall prevalence (any positive titer) of autoantibodies at the time of kidney biopsy in all patients with LN (class I-VI), any proliferative (class III, IV, III+V, or IV+V), and membranous (class V). (B-D) Autoantibody titers that significantly differ between patients with mesangial (class I), pure membranous (class V), pure proliferative (class III or IV), mixed (class III+IV or class IV+V), and advanced sclerosis (class VI) LN. Statistical significance was determined using Kruskal-Wallis test with Dunn’s multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Figure 1. Prevalence and concentrations of autoantibodies at lupus nephritis (LN) diagnosis according to LN classification. (A) Overall prevalence (any positive titer) of autoantibodies at the time of kidney biopsy in all patients with LN (class I-VI), any proliferative (class III, IV, III+V, or IV+V), and membranous (class V). (B-D) Autoantibody titers that significantly differ between patients with mesangial (class I), pure membranous (class V), pure proliferative (class III or IV), mixed (class III+IV or class IV+V), and advanced sclerosis (class VI) LN. Statistical significance was determined using Kruskal-Wallis test with Dunn’s multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
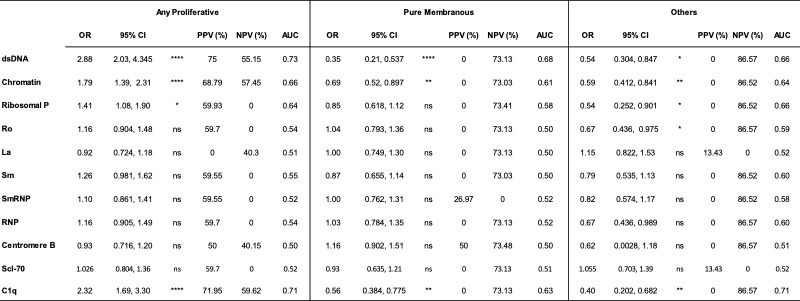 Table. Diagnostic implications of autoantibody titers at the time of kidney biopsy. For each autoantibody (rows), odds ratios (OR), positive and negative predictive values (PPV and NPV, respectively), and area under the curve (AUC) were calculated for each increase in titer by 1 standard deviation in patients with any proliferative (class III, IV, III+V or IV+V), pure membranous (class V), and other LN classes (class I, II, or VI)
Table. Diagnostic implications of autoantibody titers at the time of kidney biopsy. For each autoantibody (rows), odds ratios (OR), positive and negative predictive values (PPV and NPV, respectively), and area under the curve (AUC) were calculated for each increase in titer by 1 standard deviation in patients with any proliferative (class III, IV, III+V or IV+V), pure membranous (class V), and other LN classes (class I, II, or VI)
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1289564-3-ANY.jpg width=440 height=676.718938480096 border=0 style=border-style: none;>
Disclosures: A. Fava, Sanofi; C. Guthridge, None; J. Kheir, None; C. Wagner, None; M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; J. Buyon, None; B. Diamond, None; t. (AMP) RA/SLE, None; J. Guthridge, None; J. James, Bristol-Myers Squibb(BMS), AstraZeneca, Novartis, Progentec Biosciences.
Background/Purpose: Autoantibodies are a hallmark of lupus nephritis (LN). While there is known heterogeneity in autoantibody expression among LN patients, the association of autoantibodies with LN subtypes and the implications of longitudinal changes in LN are not entirely understood. In this study, we quantified circulating autoantibodies in the Accelerating Medicines Partnership (AMP) LN longitudinal cohort to identify novel serum biomarkers of LN classification and treatment response and to provide insights into the pathogenesis of LN.
Methods: SLE patients meeting ACR or SLICC criteria (n=268) indicated for kidney biopsy by a urine protein/creatinine (UPCR) >0.5 were recruited for this study as part of the AMP. Kidney biopsies were evaluated by a renal pathologist according to ISN/RPS classification, and serum samples were collected at the time of diagnostic kidney biopsy and 3-, 6-, and 12-months post-biopsy. Serum autoantibodies against dsDNA, chromatin, ribosomal-P, Ro, La, Sm, SmRNP, RNP, Jo-1, Scl-70, and centromere-B were measured using the BioPlex 2200® ANA kit (Bio-Rad Technologies, Hercules, CA), while anti-C1q positivity was determined by ELISA (QUANTA Lite®, Werfen, Bedford, MA). Clinical response was determined at 12 months using the Abatacept and Cyclophosphamide Combination Efficacy and Safety Study definitions for patients with ISN/RPS class III, IV, V, or a combination thereof and a baseline UPCR ratio >1.0.
Results: Most LN patients exhibited autoantibodies against chromatin (78%) dsDNA (70%), SmRNP (63%), C1q (56%), RNP (54%), and Sm (52%) (Figure 1A). Patients with proliferative LN (class III, IV, III+V, or IV+V) had higher positivity rates of several autoantibodies, including those against dsDNA, chromatin, and C1q, compared to patients with membranous LN (class V) (Figure 1A). Similarly, patients with pure proliferative (class III or IV) and mixed (class III+V or IV+V) LN had significantly higher titers of these autoantibodies compared to those with mesangial (class I or II), membranous, and advanced sclerosis (class VI) LN (Figure 1B-D). Furthermore, increased titers of these autoantibodies were associated with higher odds of having proliferative LN (Table). Proliferative LN patients with a complete treatment response exhibited a significant decline in several autoantibodies including anti-dsDNA, C1q, chromatin, Smith, and ribosomal P (Figure 2). Autoantibody levels remained relatively stable in partial- and non-responder proliferative LN patients, as well as in patients with membranous LN.
Conclusion: LN patients exhibit heterogeneous autoantibody profiles associated with ISN/RPS classification. Specifically, levels of autoantibodies against dsDNA, C1q, chromatin, and ribosomal P may serve as noninvasive biomarkers of proliferative LN. In patients with proliferative but not membranous LN, a decline in the titers of several autoantibodies, including many not routinely measured over time, such as anti-Sm, was associated with treatment response, suggesting a possible role in LN pathogenesis. In addition, these autoantibodies may serve as early biomarkers of treatment response.
.jpg) Figure 1. Prevalence and concentrations of autoantibodies at lupus nephritis (LN) diagnosis according to LN classification. (A) Overall prevalence (any positive titer) of autoantibodies at the time of kidney biopsy in all patients with LN (class I-VI), any proliferative (class III, IV, III+V, or IV+V), and membranous (class V). (B-D) Autoantibody titers that significantly differ between patients with mesangial (class I), pure membranous (class V), pure proliferative (class III or IV), mixed (class III+IV or class IV+V), and advanced sclerosis (class VI) LN. Statistical significance was determined using Kruskal-Wallis test with Dunn’s multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001
Figure 1. Prevalence and concentrations of autoantibodies at lupus nephritis (LN) diagnosis according to LN classification. (A) Overall prevalence (any positive titer) of autoantibodies at the time of kidney biopsy in all patients with LN (class I-VI), any proliferative (class III, IV, III+V, or IV+V), and membranous (class V). (B-D) Autoantibody titers that significantly differ between patients with mesangial (class I), pure membranous (class V), pure proliferative (class III or IV), mixed (class III+IV or class IV+V), and advanced sclerosis (class VI) LN. Statistical significance was determined using Kruskal-Wallis test with Dunn’s multiple comparisons. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 Table. Diagnostic implications of autoantibody titers at the time of kidney biopsy. For each autoantibody (rows), odds ratios (OR), positive and negative predictive values (PPV and NPV, respectively), and area under the curve (AUC) were calculated for each increase in titer by 1 standard deviation in patients with any proliferative (class III, IV, III+V or IV+V), pure membranous (class V), and other LN classes (class I, II, or VI)
Table. Diagnostic implications of autoantibody titers at the time of kidney biopsy. For each autoantibody (rows), odds ratios (OR), positive and negative predictive values (PPV and NPV, respectively), and area under the curve (AUC) were calculated for each increase in titer by 1 standard deviation in patients with any proliferative (class III, IV, III+V or IV+V), pure membranous (class V), and other LN classes (class I, II, or VI)<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1289564-3-ANY.jpg width=440 height=676.718938480096 border=0 style=border-style: none;>
Figure 2. Autoantibody concentrations decrease over time in patients with proliferative LN with complete treatment response. Autoantibody concentrations were measured at the time of kidney biopsy (V0) and 3 (V1), 6 (V2), and 12 (V3) months post-kidney biopsy in patients with (A) proliferative (class III, IV, III+V, or IV+V) and (B) membranous (class V) LN patients. Treatment response was determined at 12 months, with complete response (CR) defined as urine protein/creatinine (UPCR) < 0.5, serum creatinine < 125% of baseline, and prednisone < 10mg/day. Partial response (PR) was defined by the same criteria as CR, except with a UPCR >0.5 with < 50% improvement from baseline. Patients who did not meet either requirement were classified as non-responders (NR).
Disclosures: A. Fava, Sanofi; C. Guthridge, None; J. Kheir, None; C. Wagner, None; M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee; J. Buyon, None; B. Diamond, None; t. (AMP) RA/SLE, None; J. Guthridge, None; J. James, Bristol-Myers Squibb(BMS), AstraZeneca, Novartis, Progentec Biosciences.

