Back
Poster Session C
Rheumatoid arthritis (RA)
Session: (1387–1416) RA – Diagnosis, Manifestations, and Outcomes Poster III
1396: Risk of Hospitalized Infections in Older Elderly Rheumatoid Arthritis Patients Treated with Biological/Targeted Synthetic DMARDs: Evaluation Using Data from a Japanese Claims Database
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MH
Masayoshi Harigai, MD, PhD
Tokyo Women's Medical University
Tokyo, Japan
Abstract Poster Presenter(s)
Masayoshi Harigai1, Takao Fujii2, Ryoko Sakai3, Ataru Igarashi4, Ayako Shoji5, Hiroko Yamaguchi6, Katsuhiko Iwasaki6, Misako Makishima7, Amika Yoshida7, Norihiro Okada7, Katsuhisa Yamashita7 and Yutaka Kawahito8, 1Division of Rheumatology, Department of Internal Medicine, Tokyo Women’s Medical University School of Medicine, Tokyo, Japan, 2Department of Rheumatology and Clinical Immunology, Wakayama Medical University, Wakayama, Japan, 3Department of Public Health and Epidemiology, Meiji Pharmaceutical University, Tokyo, Japan, 4Department of Health Economics & Outcomes Research, Graduate School of Pharmaceutical Sciences, The University of Tokyo, Tokyo, Japan; Unit of Public Health and Preventive Medicine, Yokohama City University of Medicine, Kanagawa, Japan, 5Medilead Inc., Tokyo, Japan; Department of Health Economics and Outcomes Research, Graduate School of Pharmaceutical Sciences, University of Tokyo, Tokyo, Japan; Healthcare Consulting Inc., Tokyo, Japan, Shinjuku-ku, Japan, 6Medilead Inc., Tokyo, Japan, Shinjuku-ku, Japan, 7Chugai Pharmaceutical Co., Ltd., Chuo-ku, Tokyo, Japan, 8Inflammation and Immunology, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan, Kyoto, Kyoto, Japan
Background/Purpose: Safety evidence of biological or targeted synthetic DMARDs (b/tsDMARDs) is still insufficient in older elderly ( >=75 years old (y/o)) patients with rheumatoid arthritis (RA) , who are usually excluded from randomized clinical trials. In superaged societies, accumulation of safety data in the real world, especially those of infectious events, is one of the most pressing challenges. We therefore compared risks of hospitalized infections (HIs) across b/tsDMARDs in patients with RA in various age groups.
Methods: A retrospective longitudinal population-based study was conducted using a Japanese claims data provided by Medical Data Vision Co., Ltd. (Tokyo, Japan). We defined individuals as users of b/tsDMARDs if they met all of the following: 1) having at least one ICD10 code (M05 or M06 [excluding M06.1]); 2) having at least one prescription of b/tsDMARDs between October 10, 2014 and February 28, 2019; 3) having data prior to 12 months before the index month (baseline). The index month was defined as the first month of the prescription of b/tsDMARDs during the above term. Patients were excluded from the study population if they had a claim of at least one of the diseases except for RA with indications of b/tsDMARDs during the baseline. Patients were followed from the index month until the last exposure to b/tsDMARDs started in the index month, date of loss of follow-up, or the end of follow-up (February 2019), whichever came first. HIs were defined by ICD10 code with one prescription of predefined drugs for each infection during hospitalizations. Some of HIs were defined by ICD10 code alone. To compare the risk of HIs across b/tsDMARDs, adjusted risk ratios (aRRs) with 95% confidence intervals (95% CI) adjusted for sex, age, year of treatment start, comorbidity, glucocorticoids use, methotrexate use, and treatment duration were calculated using a Poisson regression model. Multivariable analyses were conducted in the patients ≥75 y/o, ≥65 and < 75 y/o, and < 65 y/o separately.
Results: Of 5506 patients enrolled, 2265 (41.2%), 1709 (31.0%), and 1532 (27.8%) were < 65, 65–74, and ≥75 y/o, respectively. Crude incidence rates of HIs (/100PY) were 3.99, 7.27, and 10.77 for < 65, 65–74, and ≥75 y/o, respectively. Among patients ≥75 y/o, aRRs (95% CI) of each bDMARD vs tocilizumab (TCZ) for HIs were as follows: etanercept 2.40 (1.24–4.61); adalimumab 1.90 (0.75–4.83); golimumab 1.21 (0.66–2.23); and abatacept 0.89 (0.49–1.62) (Table 1). For the analysis by drug mechanism of action, aRRs of other bDMARDs groups vs IL-6 inhibitors for HI in patients ≥75 y/o did not show a noticeable increase or decrease (Table 2). In patients < 65 years old, aRR of etanercept vs TCZ for HI was 0.30 (0.11–0.85). aRRs of other b/tsDMARDs vs TCZ for HI in patients < 65 y/o and of all b/tsDMARDs in patients aged 65–74 y/o as well as overall population did not show a noticeable increase or decrease.
Conclusion: In patients with RA ≥75 y/o, the risk of HIs in patients treated with TCZ was not different from patients treated with adalimumab, golimumab and abatacept, and lower than patients treated with etanercept.
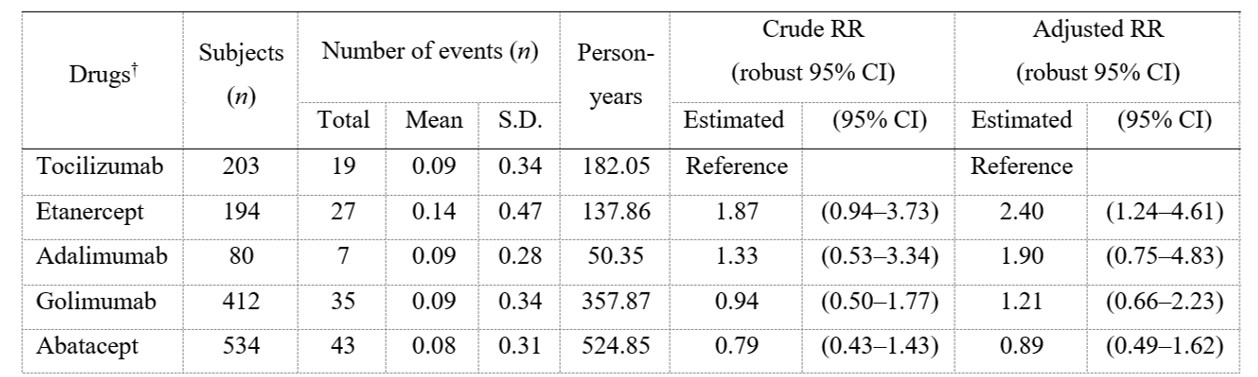 Table 1. Risk of hospitalized infections in RA patients ≥75 years old
Table 1. Risk of hospitalized infections in RA patients ≥75 years old
† b/tsDMARDs used in < 50 cases were not analyzed.
S.D, standard deviation; RR, risk ratio; 95% CI, 95% confidence interval
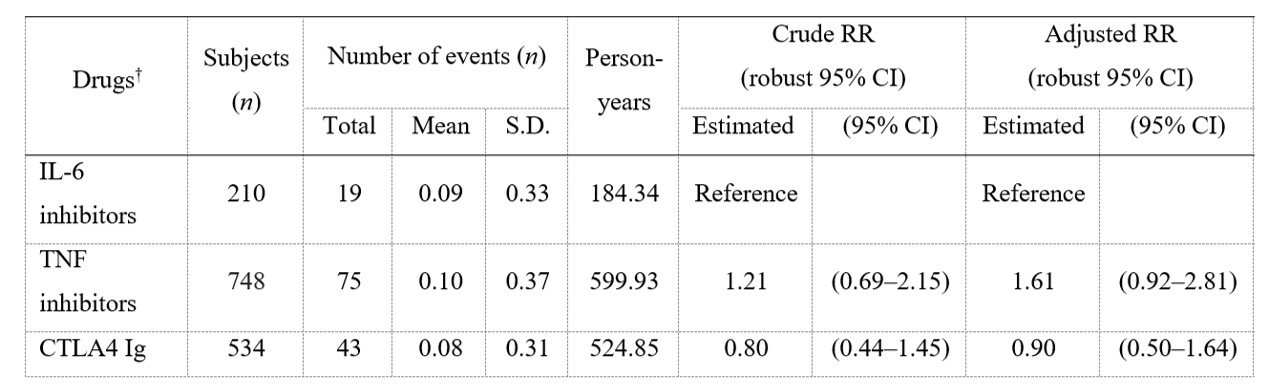 Table 2. Risk of hospitalized infections in RA patients ≥75 years old by drug mechanism of action
Table 2. Risk of hospitalized infections in RA patients ≥75 years old by drug mechanism of action
† Each b/tsDMARDs category used in < 50 cases were not analyzed.
S.D, standard deviation; RR, risk ratio; 95% CI, 95% confidence interval
Disclosures: M. Harigai, AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb(BMS), Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nippon Shinyaku Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., Viatris Japan; T. Fujii, Chugai Pharmaceutical Co., Ltd., Pfizer, Abbvie G.K., Mitsubishi Tanabe Pharma Co., Janssen Pharmaceutical K. K., Ono Pharmaceutical Co., Ltd.; R. Sakai, Chugai Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Ayumi Pharmaceutical Co., Asahi Kasei Corp., Taisho Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd.; A. Igarashi, Abbott Japan Inc, Chugai Pharmaceutical Co., Ltd., Abbvie G.K., Becton Dickinson and Company, Creative-Ceuticals Inc., Eli Lilly Japan K.K., Gilead Sciences K.K., Intuitive Surgical G.K., Milliman Inc., Pfizer Inc., Sanofi Pasteur Inc., Terumo Corporation, Astellas Pharma Inc., CSL Behring Japan Inc., FUJIFILM Corporation, Sanofi K.K., Takeda Pharmaceutical Co., Ltd.; A. Shoji, None; H. Yamaguchi, None; K. Iwasaki, None; M. Makishima, Chugai Pharmaceutical Co., Ltd.; A. Yoshida, Chugai Pharmaceutical Co., Ltd.; N. Okada, Chugai Pharmaceutical Co., Ltd.; K. Yamashita, Chugai Pharmaceutical Co., Ltd.; Y. Kawahito, Abbvie G.K., Ayumi Pharmaceutical Co., Asahi Kasei Corp., Astellas Pharma Inc., AstraZeneca, Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb(BMS), Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., GlaxoSmithKlein(GSK), Janssen Pharmaceutical K.K., Novartis International AG, Pfizer Japan Inc., Mitsubishi Tanabe Pharma Co., Nippon Shinyaku Co., Ltd., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., Torii Pharmaceutical Co., Ltd., Viatris Inc..
Background/Purpose: Safety evidence of biological or targeted synthetic DMARDs (b/tsDMARDs) is still insufficient in older elderly ( >=75 years old (y/o)) patients with rheumatoid arthritis (RA) , who are usually excluded from randomized clinical trials. In superaged societies, accumulation of safety data in the real world, especially those of infectious events, is one of the most pressing challenges. We therefore compared risks of hospitalized infections (HIs) across b/tsDMARDs in patients with RA in various age groups.
Methods: A retrospective longitudinal population-based study was conducted using a Japanese claims data provided by Medical Data Vision Co., Ltd. (Tokyo, Japan). We defined individuals as users of b/tsDMARDs if they met all of the following: 1) having at least one ICD10 code (M05 or M06 [excluding M06.1]); 2) having at least one prescription of b/tsDMARDs between October 10, 2014 and February 28, 2019; 3) having data prior to 12 months before the index month (baseline). The index month was defined as the first month of the prescription of b/tsDMARDs during the above term. Patients were excluded from the study population if they had a claim of at least one of the diseases except for RA with indications of b/tsDMARDs during the baseline. Patients were followed from the index month until the last exposure to b/tsDMARDs started in the index month, date of loss of follow-up, or the end of follow-up (February 2019), whichever came first. HIs were defined by ICD10 code with one prescription of predefined drugs for each infection during hospitalizations. Some of HIs were defined by ICD10 code alone. To compare the risk of HIs across b/tsDMARDs, adjusted risk ratios (aRRs) with 95% confidence intervals (95% CI) adjusted for sex, age, year of treatment start, comorbidity, glucocorticoids use, methotrexate use, and treatment duration were calculated using a Poisson regression model. Multivariable analyses were conducted in the patients ≥75 y/o, ≥65 and < 75 y/o, and < 65 y/o separately.
Results: Of 5506 patients enrolled, 2265 (41.2%), 1709 (31.0%), and 1532 (27.8%) were < 65, 65–74, and ≥75 y/o, respectively. Crude incidence rates of HIs (/100PY) were 3.99, 7.27, and 10.77 for < 65, 65–74, and ≥75 y/o, respectively. Among patients ≥75 y/o, aRRs (95% CI) of each bDMARD vs tocilizumab (TCZ) for HIs were as follows: etanercept 2.40 (1.24–4.61); adalimumab 1.90 (0.75–4.83); golimumab 1.21 (0.66–2.23); and abatacept 0.89 (0.49–1.62) (Table 1). For the analysis by drug mechanism of action, aRRs of other bDMARDs groups vs IL-6 inhibitors for HI in patients ≥75 y/o did not show a noticeable increase or decrease (Table 2). In patients < 65 years old, aRR of etanercept vs TCZ for HI was 0.30 (0.11–0.85). aRRs of other b/tsDMARDs vs TCZ for HI in patients < 65 y/o and of all b/tsDMARDs in patients aged 65–74 y/o as well as overall population did not show a noticeable increase or decrease.
Conclusion: In patients with RA ≥75 y/o, the risk of HIs in patients treated with TCZ was not different from patients treated with adalimumab, golimumab and abatacept, and lower than patients treated with etanercept.
 Table 1. Risk of hospitalized infections in RA patients ≥75 years old
Table 1. Risk of hospitalized infections in RA patients ≥75 years old† b/tsDMARDs used in < 50 cases were not analyzed.
S.D, standard deviation; RR, risk ratio; 95% CI, 95% confidence interval
 Table 2. Risk of hospitalized infections in RA patients ≥75 years old by drug mechanism of action
Table 2. Risk of hospitalized infections in RA patients ≥75 years old by drug mechanism of action† Each b/tsDMARDs category used in < 50 cases were not analyzed.
S.D, standard deviation; RR, risk ratio; 95% CI, 95% confidence interval
Disclosures: M. Harigai, AbbVie Japan GK, Asahi Kasei Corp., Astellas Pharma Inc., Ayumi Pharmaceutical Co., Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb(BMS), Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., Kaken Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Co., Mochida Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Nippon Shinyaku Co., Ltd., Pfizer Japan Inc., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., UCB Japan Co., Ltd., Viatris Japan; T. Fujii, Chugai Pharmaceutical Co., Ltd., Pfizer, Abbvie G.K., Mitsubishi Tanabe Pharma Co., Janssen Pharmaceutical K. K., Ono Pharmaceutical Co., Ltd.; R. Sakai, Chugai Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Nippon Kayaku Co., Ltd., Ayumi Pharmaceutical Co., Asahi Kasei Corp., Taisho Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd.; A. Igarashi, Abbott Japan Inc, Chugai Pharmaceutical Co., Ltd., Abbvie G.K., Becton Dickinson and Company, Creative-Ceuticals Inc., Eli Lilly Japan K.K., Gilead Sciences K.K., Intuitive Surgical G.K., Milliman Inc., Pfizer Inc., Sanofi Pasteur Inc., Terumo Corporation, Astellas Pharma Inc., CSL Behring Japan Inc., FUJIFILM Corporation, Sanofi K.K., Takeda Pharmaceutical Co., Ltd.; A. Shoji, None; H. Yamaguchi, None; K. Iwasaki, None; M. Makishima, Chugai Pharmaceutical Co., Ltd.; A. Yoshida, Chugai Pharmaceutical Co., Ltd.; N. Okada, Chugai Pharmaceutical Co., Ltd.; K. Yamashita, Chugai Pharmaceutical Co., Ltd.; Y. Kawahito, Abbvie G.K., Ayumi Pharmaceutical Co., Asahi Kasei Corp., Astellas Pharma Inc., AstraZeneca, Boehringer Ingelheim Japan, Inc., Bristol-Myers Squibb(BMS), Chugai Pharmaceutical Co., Ltd., Daiichi-Sankyo, Inc., Eisai Co., Ltd., Eli Lilly Japan K.K., GlaxoSmithKlein(GSK), Janssen Pharmaceutical K.K., Novartis International AG, Pfizer Japan Inc., Mitsubishi Tanabe Pharma Co., Nippon Shinyaku Co., Ltd., Taisho Pharmaceutical Co., Ltd., Teijin Pharma Ltd., Torii Pharmaceutical Co., Ltd., Viatris Inc..

