Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0285: Characteristics and Outcomes of Patients with Rheumatoid Arthritis Treated with Upadacitinib in a Global Real-World Setting
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- RC
Roberto Caporali, MD
Universita statale di Milano
Milano, Milan, Italy
Abstract Poster Presenter(s)
Roberto Felice Caporali1, Jayeshkumar Patel2, Oliver Howell3, sander strengholt4, Hannah Jones3 and Peter Taylor5, 1University of Milan, Milano, Italy, 2AbbVie, Inc., North Chicago, IL, 3Adelphi Real World, Bollington, United Kingdom, 4AbbVie, Inc., Abcoude, Netherlands, 5University of Oxford, Oxford, United Kingdom
Background/Purpose: Upadacitinib (UPA), a Janus kinase inhibitor, is a newly approved advanced therapy for patients (pts) with rheumatoid arthritis (RA), thus real-world evidence is lacking. The objective of this analysis was to describe the characteristics and outcomes of pts with RA initiating UPA in real-world clinical practice in the EU, Canada, and Japan.
Methods: This study utilized data from the Adelphi RA Disease Specific Programme™, a point-in-time survey administered to rheumatologists and pts from July 2021 to February 2022. Rheumatologists from Germany, France, Italy, Spain, the UK, Canada, and Japan were included in this analysis. Rheumatologists (n=410) completed online patient record forms for 746 RA pts who received UPA for ≥6 months; eligible pts had a ≥12-month treatment history prior to UPA initiation (baseline). Physicians and pts provided data on pts demographic and clinical characteristics, treatment history, and disease outcomes. Findings reported here include physician-reported disease activity (within the following DAS28 boundaries: inactive = < 2.6; low = 2.6–3.2; moderate = 3.3–5.1; high = >5.6), pain, tender/swollen joint counts at UPA initiation and the most recent follow-up (≥6 months from UPA initiation), motivations for choosing UPA, and treatment satisfaction. Patient-reported treatment satisfaction, fatigue (Functional Assessment of Chronic Illness Therapy – Fatigue [FACIT-F]), and functional status (Health Assessment Questionnaire – Disability Index [HAQ-DI]) are also reported.
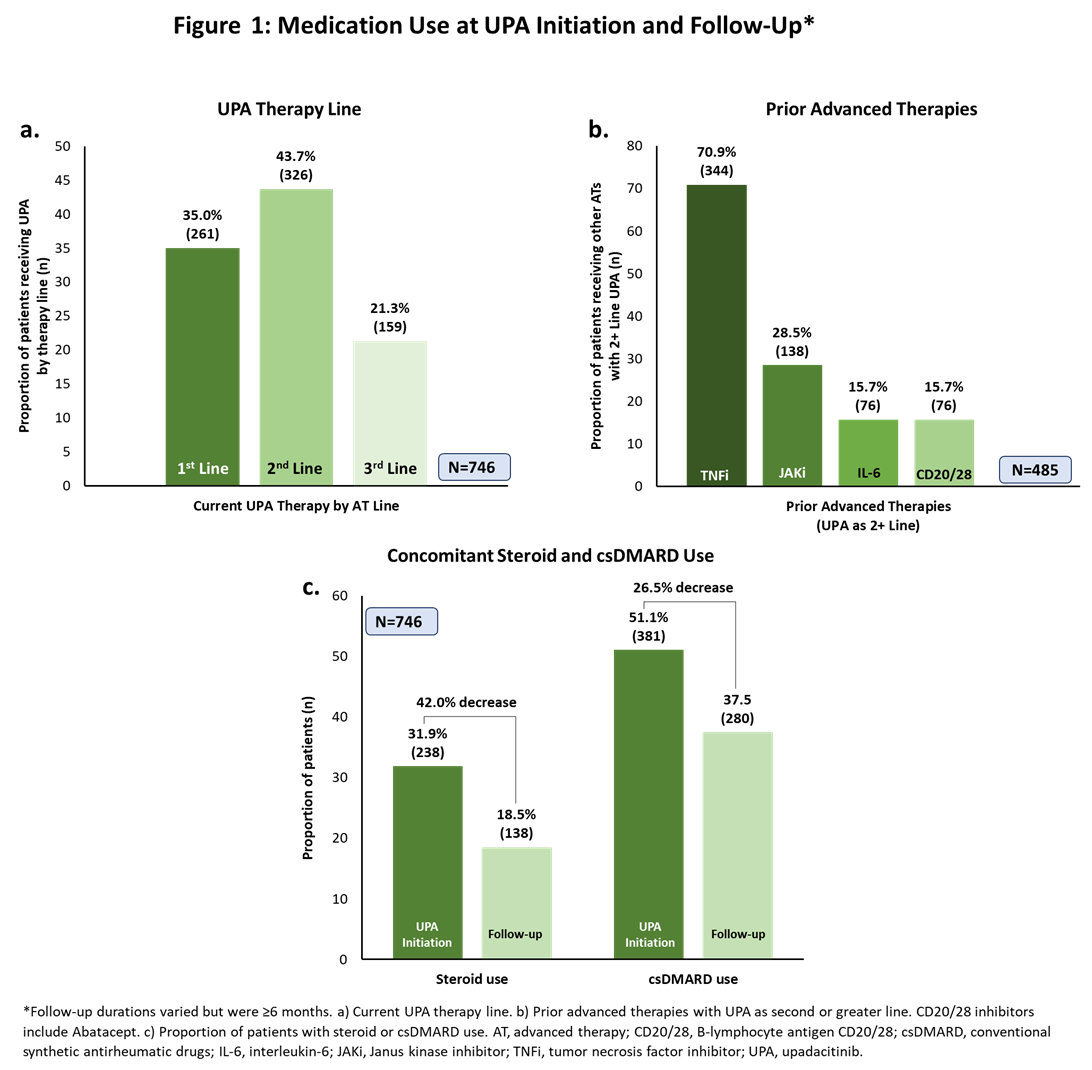
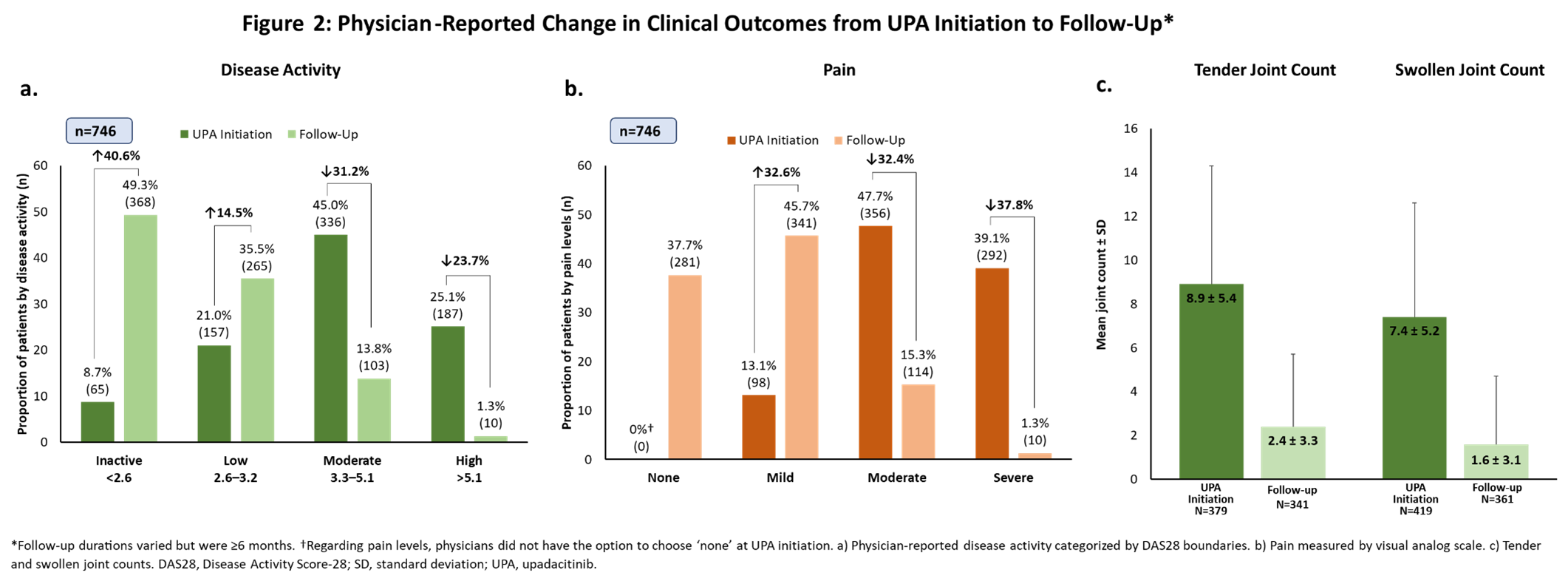
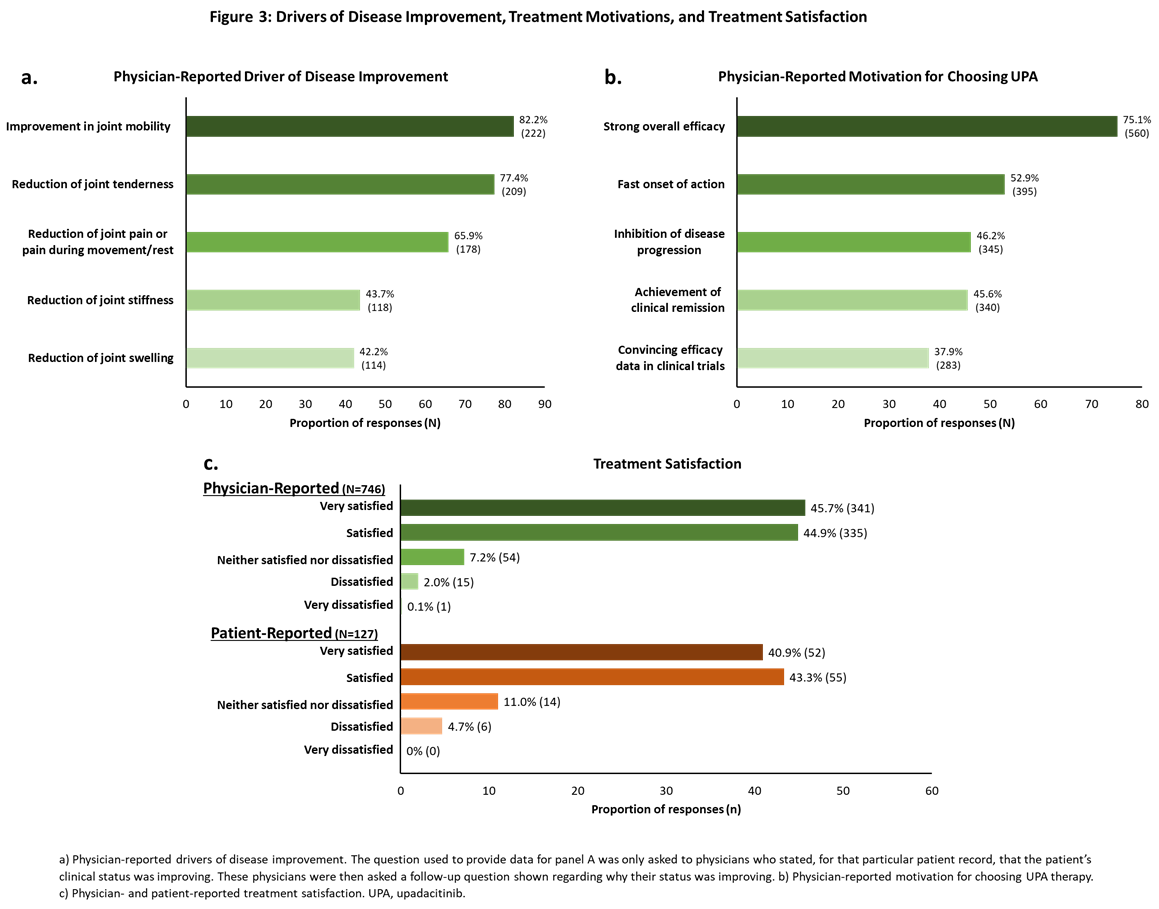
Results: The mean (standard deviation [SD]) age of pts included in the analysis was 54.4 ± 11.8 years with a mean time since diagnosis of 6.8 ± 5.8 years; 67.0% were female. Over a third of pts (35.0%) received UPA as 1st line advanced therapy; among those taking UPA as ≥2nd line therapy, 70.9% had received a TNFi (Figure 1a-b). Compared to baseline, fewer pts continued use of steroids and conventional synthetic DMARDs (Figure 1c) at follow-up. At UPA initiation, most pts had moderate/high (45.0%/25.1%) disease activity and moderate/severe (47.7%/39.1%) pain (Figure 2a-b). At follow-up, 49.3% had inactive disease and >83% reported mild or no pain. Patients also had marked reductions from baseline in tender and swollen joint counts at follow-up (Figure 2c). Fifty-seven percent of pts achieved normative values in FACIT-F (≥40.1) and 30% achieved normative HAQ-DI scores (≤0.25). Improvement in joint mobility and reduction of joint tenderness or joint pain during movement or rest were identified by physicians as the drivers of disease improvement (Figure 3a). Moreover, physicians reported strong overall efficacy and fast onset of action as reasons for prescribing UPA (Figure 3b). Most physicians were satisfied (44.9%) or very satisfied (45.7%) with the current treatment, as were most pts (satisfied: 43.3%; very satisfied: 40.9%; Figure 3c).
Conclusion: This analysis of real-world data in European, Canadian, and Japanese pts with RA demonstrated that pts receiving UPA for ≥6 months have improved disease and pain severity, joint tenderness, and joint mobility. Overall, most physicians and pts were satisfied or very satisfied with current disease control with UPA.
Disclosures: R. Caporali, AbbVie, Celltrion, Fresenius-Kabi, Galapagos, Janssen, Pfizer, Roche, UCB, Eli Lilly, Gilead, Sanofi; J. Patel, AbbVie; O. Howell, Adelphi Real World, AbbVie; s. strengholt, AbbVie; H. Jones, Adelphi Real World, AbbVie; P. Taylor, Biogen, Celltrion, Eli Lilly, Fresenius Kabi, Gilead, GlaxoSmithKlein(GSK), Janssen, Nordic Pharma, Pfizer, Roche, Sanofi, UCB, Galapagos, Abbvie.
Background/Purpose: Upadacitinib (UPA), a Janus kinase inhibitor, is a newly approved advanced therapy for patients (pts) with rheumatoid arthritis (RA), thus real-world evidence is lacking. The objective of this analysis was to describe the characteristics and outcomes of pts with RA initiating UPA in real-world clinical practice in the EU, Canada, and Japan.
Methods: This study utilized data from the Adelphi RA Disease Specific Programme™, a point-in-time survey administered to rheumatologists and pts from July 2021 to February 2022. Rheumatologists from Germany, France, Italy, Spain, the UK, Canada, and Japan were included in this analysis. Rheumatologists (n=410) completed online patient record forms for 746 RA pts who received UPA for ≥6 months; eligible pts had a ≥12-month treatment history prior to UPA initiation (baseline). Physicians and pts provided data on pts demographic and clinical characteristics, treatment history, and disease outcomes. Findings reported here include physician-reported disease activity (within the following DAS28 boundaries: inactive = < 2.6; low = 2.6–3.2; moderate = 3.3–5.1; high = >5.6), pain, tender/swollen joint counts at UPA initiation and the most recent follow-up (≥6 months from UPA initiation), motivations for choosing UPA, and treatment satisfaction. Patient-reported treatment satisfaction, fatigue (Functional Assessment of Chronic Illness Therapy – Fatigue [FACIT-F]), and functional status (Health Assessment Questionnaire – Disability Index [HAQ-DI]) are also reported.
Results: The mean (standard deviation [SD]) age of pts included in the analysis was 54.4 ± 11.8 years with a mean time since diagnosis of 6.8 ± 5.8 years; 67.0% were female. Over a third of pts (35.0%) received UPA as 1st line advanced therapy; among those taking UPA as ≥2nd line therapy, 70.9% had received a TNFi (Figure 1a-b). Compared to baseline, fewer pts continued use of steroids and conventional synthetic DMARDs (Figure 1c) at follow-up. At UPA initiation, most pts had moderate/high (45.0%/25.1%) disease activity and moderate/severe (47.7%/39.1%) pain (Figure 2a-b). At follow-up, 49.3% had inactive disease and >83% reported mild or no pain. Patients also had marked reductions from baseline in tender and swollen joint counts at follow-up (Figure 2c). Fifty-seven percent of pts achieved normative values in FACIT-F (≥40.1) and 30% achieved normative HAQ-DI scores (≤0.25). Improvement in joint mobility and reduction of joint tenderness or joint pain during movement or rest were identified by physicians as the drivers of disease improvement (Figure 3a). Moreover, physicians reported strong overall efficacy and fast onset of action as reasons for prescribing UPA (Figure 3b). Most physicians were satisfied (44.9%) or very satisfied (45.7%) with the current treatment, as were most pts (satisfied: 43.3%; very satisfied: 40.9%; Figure 3c).
Conclusion: This analysis of real-world data in European, Canadian, and Japanese pts with RA demonstrated that pts receiving UPA for ≥6 months have improved disease and pain severity, joint tenderness, and joint mobility. Overall, most physicians and pts were satisfied or very satisfied with current disease control with UPA.



Disclosures: R. Caporali, AbbVie, Celltrion, Fresenius-Kabi, Galapagos, Janssen, Pfizer, Roche, UCB, Eli Lilly, Gilead, Sanofi; J. Patel, AbbVie; O. Howell, Adelphi Real World, AbbVie; s. strengholt, AbbVie; H. Jones, Adelphi Real World, AbbVie; P. Taylor, Biogen, Celltrion, Eli Lilly, Fresenius Kabi, Gilead, GlaxoSmithKlein(GSK), Janssen, Nordic Pharma, Pfizer, Roche, Sanofi, UCB, Galapagos, Abbvie.

