Back
Poster Session C
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: (1518–1542) Systemic Sclerosis and Related Disorders – Clinical Poster II
1522: The Collaborative National Quality and Efficacy Registry for Scleroderma: Association of Medication Use on Gastrointestinal Tract Symptoms in Early Disease
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SL
Sarah Luebker, DO
Vanderbilt Medical University Cennter
Nashville, TN, United States
Abstract Poster Presenter(s)
Sarah Luebker1, Tracy Frech1, Shervin Assassi2, Jessica Gordon3, Elana Bernstein4, Virginia Steen5, Ami Shah6, Laura Hummers7, Carrie Richardson8, Dinesh Khanna9, Flavia Castelino10, Lorinda Chung11, Faye Hant12, Vicki Shanmugam13, John VanBuren14, Jessica Alvey14, Monica Harding14, Luke Evnin15 and Nora Sandorfi16, 1Vanderbilt University Medical Center, Nashville, TN, 2McGovern Medical School, University of Texas, Houston, TX, 3Hospital for Special Surgery, New York, NY, 4Columbia University, New York, NY, 5Georgetown University School of Medicine, Washington, DC, 6Johns Hopkins Rheumatology, Baltimore, MD, 7Johns Hopkins Univerisity, Baltimore, MD, 8Northwestern University, Chicago, IL, 9Division of Rheumatology, Department of Internal Medicine, Scleroderma Program, University of Michigan, Ann Arbor, MI, 10Massachusetts General Hospital, Boston, MA, 11Stanford University, Stanford, CA, 12Medical University of South Carolina, Charleston, SC, 13George Washington University, Washington, DC, 14University of Utah, Salt Lake City, UT, 15Scleroderma Research Foundation, Brisbane, CA, 16University of Pennsylvania, Philadelphia, PA
Background/Purpose: Gastrointestinal tract (GIT) symptoms are common amongst systemic sclerosis (SSc) patients.1 The Scleroderma Clinical Trials Consortium University of California Los Angeles Gastrointestinal Tract Questionnaire (GIT 2.0) allows a clinician to assess the preceding seven days of reflux, bloating/distention, diarrhea, constipation, soilage as well as the emotional and social impact of gastrointestinal symptoms into an absent-to- mild, moderate, or severe categorization, which can be trended over subsequent care visits.2,3 The Collaborative National Quality and Efficacy Registry (CONQUER) is an effort in the United States to collect data on state-of-the-art clinical practice at SSc centers of excellence in patients with < 5 years disease duration.4 In this study, we describe the SSc gastrointestinal tract natural history and association to medication use in CONQUER participants.
Methods: The inclusion criteria for this project included CONQUER participants that had completed a minimum of two serial GIT 2.0. Patients were categorized by total GIT 2.0 severity and subsequent categories were created: no change (none-to-mild), improvement in category, worsening in category, and no change (moderate-to-severe). Clinical features including demographics, disease duration, cutaneous subset, and autoantibodies as well as medication changes between care visits were examined in each of these categories. Medications were categorized as gastrointestinal tract targeted therapy, anti-fibrotic (nintedanib only), immunosuppression, or immunomodulatory drugs (hydroxychloroquine only). Analysis included a proportional odds model accounting for linear and mixed effects of described variables.
Results: At the time of data analysis on May 4, 2022, there were 399 enrolled CONQUER participants that met project inclusion criteria. The sociodemographic and disease characteristics of those 399 participants categorized by change in GIT symptoms are shown in Table 1. Most participants (n=208, 52%) had stable mild GIT symptoms at all observed clinical assessments. The timing of medication initiation in relationship to GIT survey administration is shown in Figure 1. On average, patients that had a change did so within the first year of enrollment in CONQUER. In all GIT categories, reflux medication and immunosuppression initiation preceded baseline visit whereas anti-fibrotic initiation occurred at or after baseline visit. There were no patients on IVIG or pirfenidone. The odds of having a worse GIT score at any visit is shown in Table 3 with significance found in weight loss, tobacco use, or the use of reflux or promotility medication.
Conclusion: This report from the CONQUER cohort, suggests that the majority of early patients have stable mild GIT disease. There is no clear association of immunosuppression or anti-fibrotic use with worsening GIT symptoms, but tobacco use is clearly associated, highlighting the importance of smoking cessation.
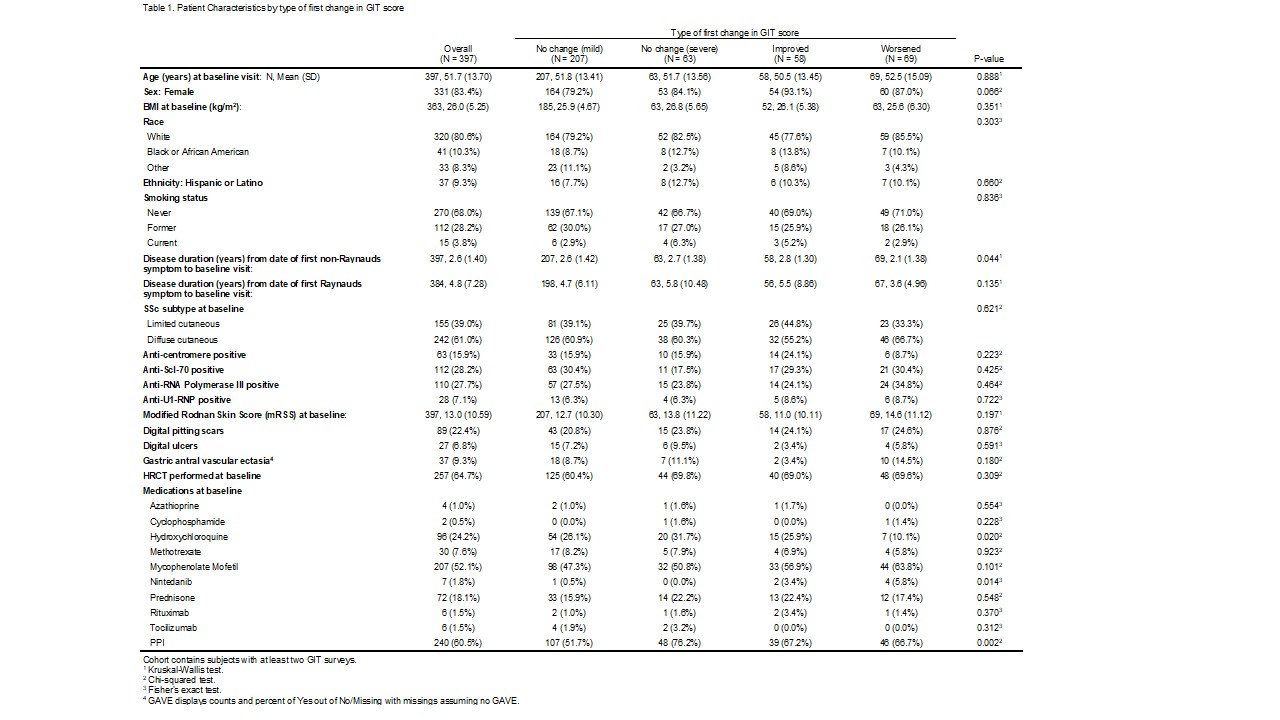 Table 1: Patient Characteristics by first change in GIT Score
Table 1: Patient Characteristics by first change in GIT Score
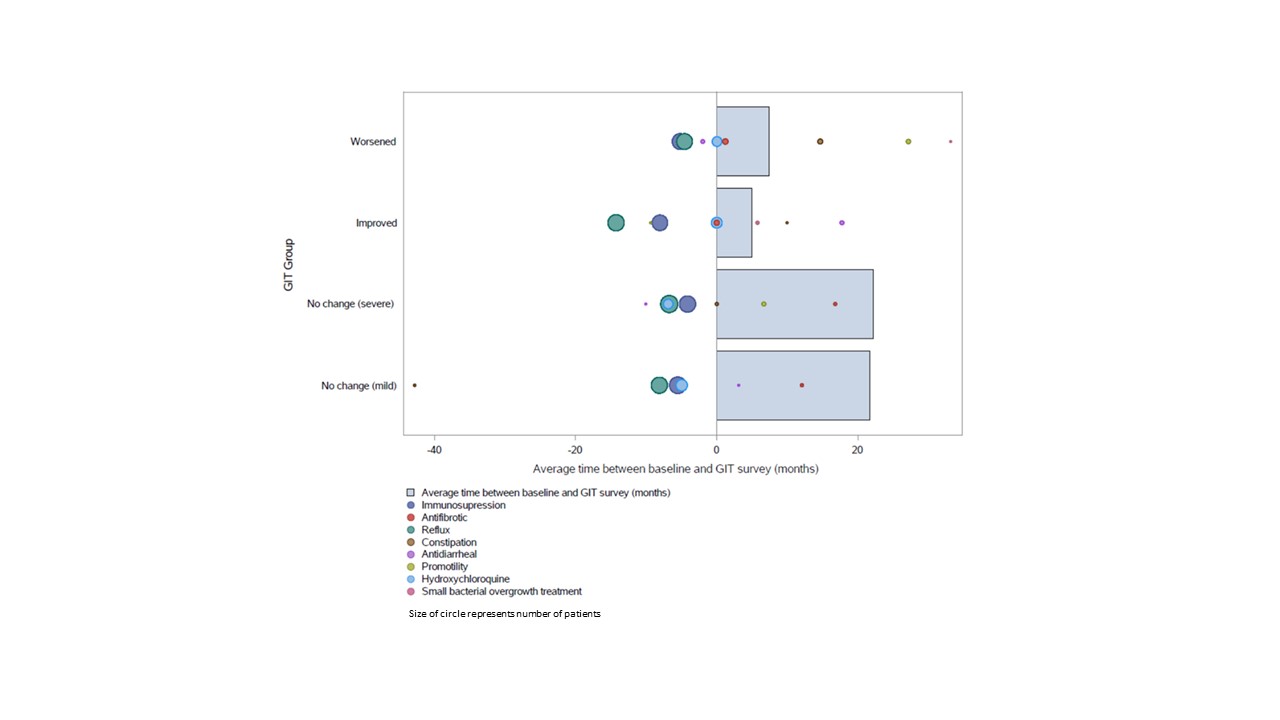 Figure 1: Timing of Medication Initiation in Each GIT Severity Group
Figure 1: Timing of Medication Initiation in Each GIT Severity Group
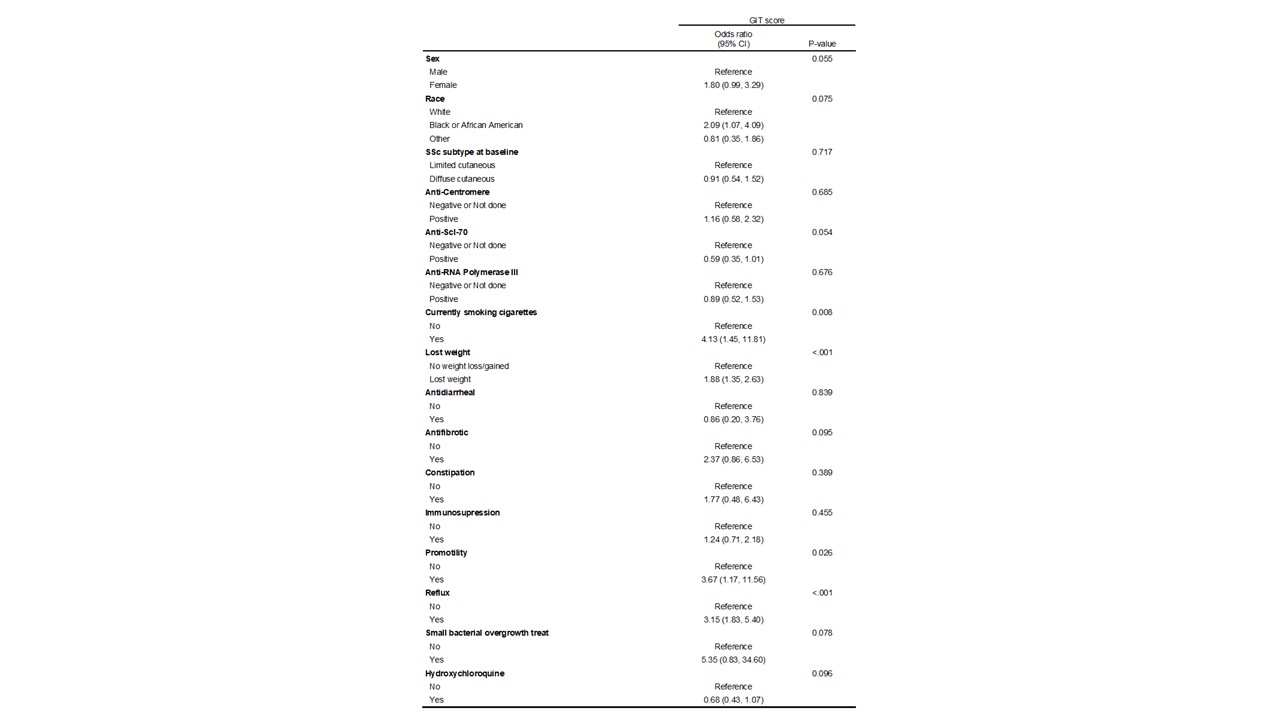 Table 2: Proportional odds model - modeling the odds of having a worse GIT score
Table 2: Proportional odds model - modeling the odds of having a worse GIT score
Disclosures: S. Luebker, None; T. Frech, None; S. Assassi, Boehringer-Ingelheim, Janssen, Novartis, AstraZeneca, CSL Behring, AbbVie/Abbott; J. Gordon, None; E. Bernstein, None; V. Steen, None; A. Shah, Arena Pharmaceuticals, Medpace/Eicos, Kadmon Corporation; L. Hummers, Boehringer-Ingelheim, Corbus Pharmaceuticals, Cumberland Pharmaceuticals, Kadmon Corporation, Medpace, CSL Behring, Mitsubishi Tanabe, Horizon Pharmaceuticals; C. Richardson, None; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences; F. Castelino, Boehringer-Ingelheim; L. Chung, None; F. Hant, None; V. Shanmugam, None; J. VanBuren, None; J. Alvey, None; M. Harding, None; L. Evnin, None; N. Sandorfi, None.
Background/Purpose: Gastrointestinal tract (GIT) symptoms are common amongst systemic sclerosis (SSc) patients.1 The Scleroderma Clinical Trials Consortium University of California Los Angeles Gastrointestinal Tract Questionnaire (GIT 2.0) allows a clinician to assess the preceding seven days of reflux, bloating/distention, diarrhea, constipation, soilage as well as the emotional and social impact of gastrointestinal symptoms into an absent-to- mild, moderate, or severe categorization, which can be trended over subsequent care visits.2,3 The Collaborative National Quality and Efficacy Registry (CONQUER) is an effort in the United States to collect data on state-of-the-art clinical practice at SSc centers of excellence in patients with < 5 years disease duration.4 In this study, we describe the SSc gastrointestinal tract natural history and association to medication use in CONQUER participants.
Methods: The inclusion criteria for this project included CONQUER participants that had completed a minimum of two serial GIT 2.0. Patients were categorized by total GIT 2.0 severity and subsequent categories were created: no change (none-to-mild), improvement in category, worsening in category, and no change (moderate-to-severe). Clinical features including demographics, disease duration, cutaneous subset, and autoantibodies as well as medication changes between care visits were examined in each of these categories. Medications were categorized as gastrointestinal tract targeted therapy, anti-fibrotic (nintedanib only), immunosuppression, or immunomodulatory drugs (hydroxychloroquine only). Analysis included a proportional odds model accounting for linear and mixed effects of described variables.
Results: At the time of data analysis on May 4, 2022, there were 399 enrolled CONQUER participants that met project inclusion criteria. The sociodemographic and disease characteristics of those 399 participants categorized by change in GIT symptoms are shown in Table 1. Most participants (n=208, 52%) had stable mild GIT symptoms at all observed clinical assessments. The timing of medication initiation in relationship to GIT survey administration is shown in Figure 1. On average, patients that had a change did so within the first year of enrollment in CONQUER. In all GIT categories, reflux medication and immunosuppression initiation preceded baseline visit whereas anti-fibrotic initiation occurred at or after baseline visit. There were no patients on IVIG or pirfenidone. The odds of having a worse GIT score at any visit is shown in Table 3 with significance found in weight loss, tobacco use, or the use of reflux or promotility medication.
Conclusion: This report from the CONQUER cohort, suggests that the majority of early patients have stable mild GIT disease. There is no clear association of immunosuppression or anti-fibrotic use with worsening GIT symptoms, but tobacco use is clearly associated, highlighting the importance of smoking cessation.
 Table 1: Patient Characteristics by first change in GIT Score
Table 1: Patient Characteristics by first change in GIT Score Figure 1: Timing of Medication Initiation in Each GIT Severity Group
Figure 1: Timing of Medication Initiation in Each GIT Severity Group Table 2: Proportional odds model - modeling the odds of having a worse GIT score
Table 2: Proportional odds model - modeling the odds of having a worse GIT scoreDisclosures: S. Luebker, None; T. Frech, None; S. Assassi, Boehringer-Ingelheim, Janssen, Novartis, AstraZeneca, CSL Behring, AbbVie/Abbott; J. Gordon, None; E. Bernstein, None; V. Steen, None; A. Shah, Arena Pharmaceuticals, Medpace/Eicos, Kadmon Corporation; L. Hummers, Boehringer-Ingelheim, Corbus Pharmaceuticals, Cumberland Pharmaceuticals, Kadmon Corporation, Medpace, CSL Behring, Mitsubishi Tanabe, Horizon Pharmaceuticals; C. Richardson, None; D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences; F. Castelino, Boehringer-Ingelheim; L. Chung, None; F. Hant, None; V. Shanmugam, None; J. VanBuren, None; J. Alvey, None; M. Harding, None; L. Evnin, None; N. Sandorfi, None.

