Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0343–0371) SLE – Treatment Poster I
0354: Clinical and Economic Characterization of Systemic Lupus Erythematosus Patients by Cumulative Corticosteroid Dose over 1 Year: Real-World Observation of Commercially Insured Adults in the United States
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SW
Sandra Wu, PhD
AstraZeneca
Hockessin, DE, United States
Abstract Poster Presenter(s)
Sandra Sze-jung Wu1, Allison Perry2, Helen Varker3, Rich Bizier3, Liisa Palmer3 and Gary Bryant4, 1AstraZeneca, Hockessin, DE, 2IBM Watson Health, New York, NY, 3Merative, Cambridge, MA, 4AstraZeneca, New Castle, DE
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex, heterogeneous disease associated with periods of flares. Identifying patients at risk of severe disease and associated resource burden is challenging. While corticosteroids are a mainstay of SLE treatment, dosing regimens are based on limited data and associated with clinical challenges. Identifying the relationship between corticosteroid dosing and burden of illness may aid in identifying high-risk SLE patients.
To quantify flares, comorbidities and resource utilization/expenditures among a prevalent cohort of patients with SLE based on cumulative oral corticosteroid dose over 1 year.
Methods: Adults with SLE were selected from IBM MarketScan Commercial Database (2013-2020) based on ≥1 inpatient (IP) claim with SLE diagnosis, or ≥2 non-diagnostic SLE outpatient (OP) claims with ≥1 rheumatologist/nephrologist visit. Index date was randomly selected among claims after the initial SLE diagnosis to create a prevalent cohort. Additional criteria included continuous medical and pharmacy benefits 12 months pre-index (baseline) and post-index, and valid steroid dosage values in the claims. SLE flares were defined using published algorithm of SLE-related treatment and diagnoses.1 Total quantity supplied of prednisone-equivalent dose (PED) of oral corticosteroid (OCS) were calculated during baseline, and patients were categorized into mutually exclusive groups based on absence of OCS (N-OCS) and the four OCS quartiles.
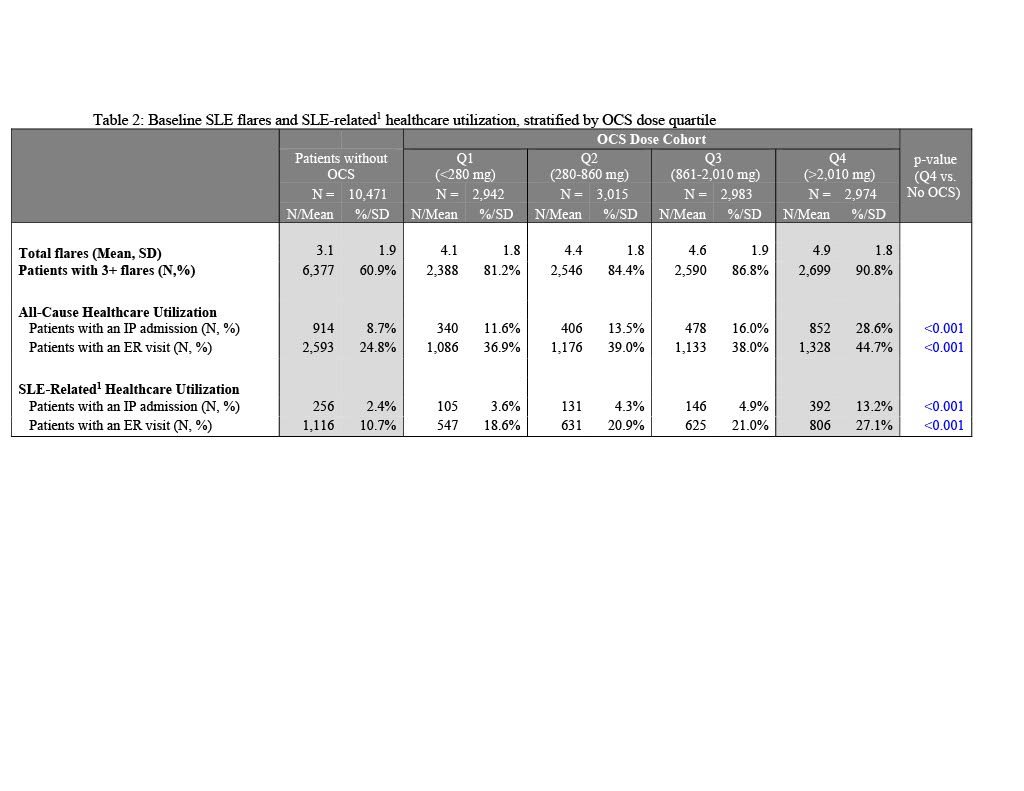
Results: Of 22,385 patients, 47% had no PED OCS. Cumulative dose (mg/year) quartile cutoffs were Q1: < 280; Q2: 280-860; Q3: 861-2,010; Q4: >2,010. Mean[SD] Elixhauser Comorbidity Index was highest in Q4 cohort (4.4[9.5]) and lowest in Q1 cohort (0.6[6.3]); scores for Q4 cohort were significantly higher than N-OCS (p< 0.001). A majority of SLE-related comorbidities were significantly higher in Q4 vs. N-OCS. More than half of Q4 cohort had cardiovascular disease (50.9%), an antimalarial (72.6%), or immunosuppressant (69.0%). Mean number of flares was highest in patients in the Q4 cohort (4.9[1.8]) with >90% of patients experiencing 3+ flares. The Q4 cohort had high rates of all-cause IP admissions (28.6% vs. 8.7-16.0%), SLE-related IP admissions (13.2% vs. 2.4-4.9%), all-cause emergency room(ER) visits (44.7% vs. 24.8-39.0%) and SLE-related ER visits (27.1% vs. 10.7-21.0%) relative to all other OCS cohorts. The Q4 cohort was associated with highest all-cause total healthcare costs ($62,848[$126,451]) and SLE-related costs ($22,111[$42,234]), three times higher than those of N-OCS cohort. SLE-related inpatient costs for the Q4 cohort were $15,870, 6 times higher than the N-OCS cohort. Drivers of SLE-related costs in Q4 cohort were OP services (53.3%) and IP costs (27.4%).
Conclusion: SLE patients in the highest quartile of total OCS dose have particularly high rates of comorbidities, flares, and utilization/costs. Results demonstrate a disproportionate burden and potential unmet need in the SLE treatment landscape, particularly for patients treated with high OCS doses who may benefit most from improved care options.
1. Garris C, et al. J Med Econ. 2013;16(5):667-677.
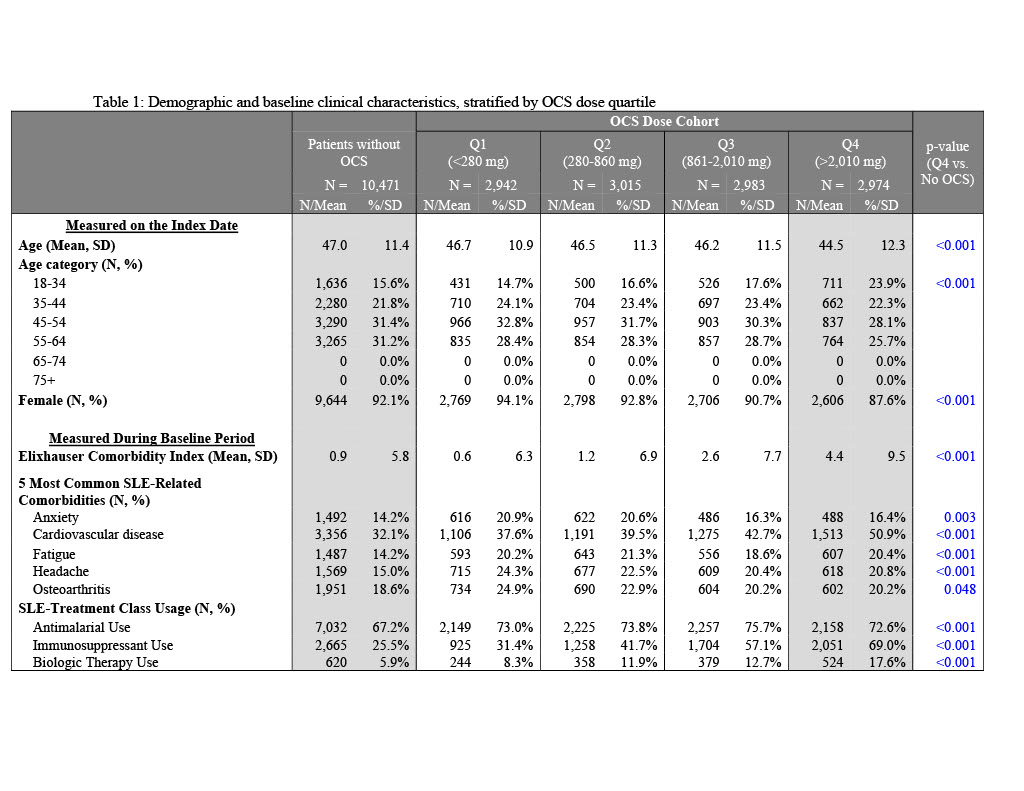
 1Comprehensive SLE-related utilization and costs included and IP or ER claims with an SLE diagnosis, as well as any IP and ER claims for SLE-related comorbidities based on Garris C, Shah M, Farrelly E. Cost Eff Resour Alloc. 2015;13:9.
1Comprehensive SLE-related utilization and costs included and IP or ER claims with an SLE diagnosis, as well as any IP and ER claims for SLE-related comorbidities based on Garris C, Shah M, Farrelly E. Cost Eff Resour Alloc. 2015;13:9.
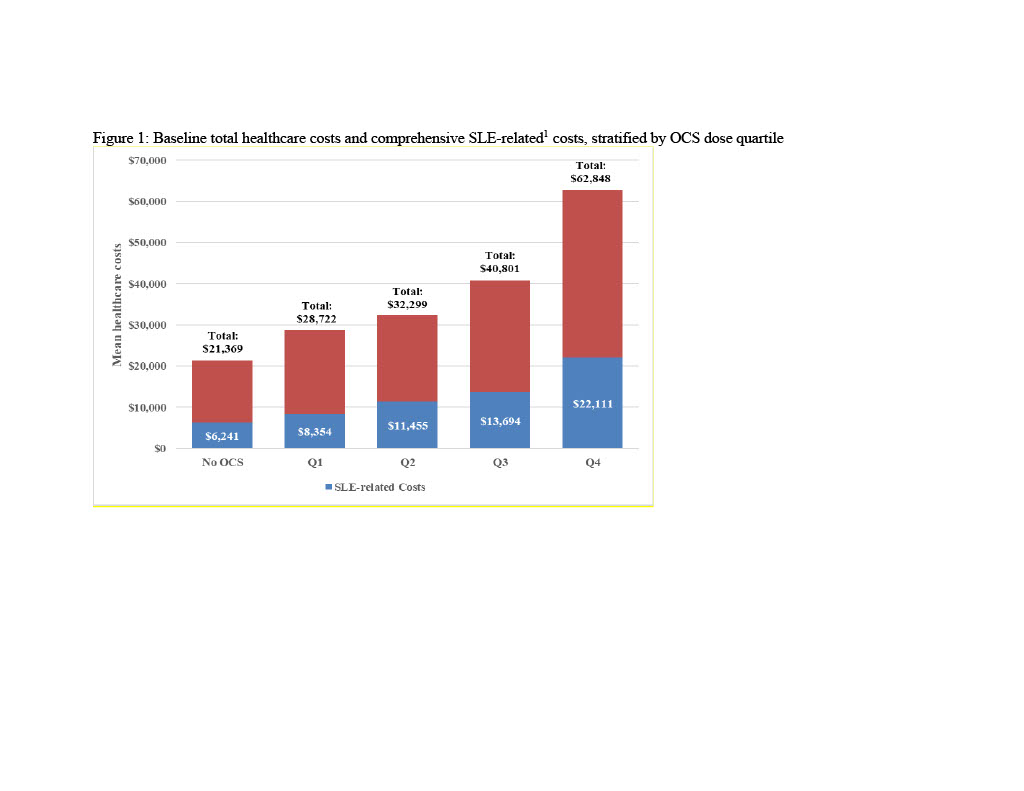 1Comprehensive SLE-related utilization and costs included and IP or ER claims with an SLE diagnosis, as well as any IP and ER claims for SLE-related comorbidities based on Garris C, Shah M, Farrelly E. Cost Eff Resour Alloc. 2015;13:9.
1Comprehensive SLE-related utilization and costs included and IP or ER claims with an SLE diagnosis, as well as any IP and ER claims for SLE-related comorbidities based on Garris C, Shah M, Farrelly E. Cost Eff Resour Alloc. 2015;13:9.
Disclosures: S. Wu, AstraZeneca; A. Perry, None; H. Varker, None; R. Bizier, None; L. Palmer, None; G. Bryant, AstraZeneca, AstraZeneca.
Background/Purpose: Systemic lupus erythematosus (SLE) is a complex, heterogeneous disease associated with periods of flares. Identifying patients at risk of severe disease and associated resource burden is challenging. While corticosteroids are a mainstay of SLE treatment, dosing regimens are based on limited data and associated with clinical challenges. Identifying the relationship between corticosteroid dosing and burden of illness may aid in identifying high-risk SLE patients.
To quantify flares, comorbidities and resource utilization/expenditures among a prevalent cohort of patients with SLE based on cumulative oral corticosteroid dose over 1 year.
Methods: Adults with SLE were selected from IBM MarketScan Commercial Database (2013-2020) based on ≥1 inpatient (IP) claim with SLE diagnosis, or ≥2 non-diagnostic SLE outpatient (OP) claims with ≥1 rheumatologist/nephrologist visit. Index date was randomly selected among claims after the initial SLE diagnosis to create a prevalent cohort. Additional criteria included continuous medical and pharmacy benefits 12 months pre-index (baseline) and post-index, and valid steroid dosage values in the claims. SLE flares were defined using published algorithm of SLE-related treatment and diagnoses.1 Total quantity supplied of prednisone-equivalent dose (PED) of oral corticosteroid (OCS) were calculated during baseline, and patients were categorized into mutually exclusive groups based on absence of OCS (N-OCS) and the four OCS quartiles.
Results: Of 22,385 patients, 47% had no PED OCS. Cumulative dose (mg/year) quartile cutoffs were Q1: < 280; Q2: 280-860; Q3: 861-2,010; Q4: >2,010. Mean[SD] Elixhauser Comorbidity Index was highest in Q4 cohort (4.4[9.5]) and lowest in Q1 cohort (0.6[6.3]); scores for Q4 cohort were significantly higher than N-OCS (p< 0.001). A majority of SLE-related comorbidities were significantly higher in Q4 vs. N-OCS. More than half of Q4 cohort had cardiovascular disease (50.9%), an antimalarial (72.6%), or immunosuppressant (69.0%). Mean number of flares was highest in patients in the Q4 cohort (4.9[1.8]) with >90% of patients experiencing 3+ flares. The Q4 cohort had high rates of all-cause IP admissions (28.6% vs. 8.7-16.0%), SLE-related IP admissions (13.2% vs. 2.4-4.9%), all-cause emergency room(ER) visits (44.7% vs. 24.8-39.0%) and SLE-related ER visits (27.1% vs. 10.7-21.0%) relative to all other OCS cohorts. The Q4 cohort was associated with highest all-cause total healthcare costs ($62,848[$126,451]) and SLE-related costs ($22,111[$42,234]), three times higher than those of N-OCS cohort. SLE-related inpatient costs for the Q4 cohort were $15,870, 6 times higher than the N-OCS cohort. Drivers of SLE-related costs in Q4 cohort were OP services (53.3%) and IP costs (27.4%).
Conclusion: SLE patients in the highest quartile of total OCS dose have particularly high rates of comorbidities, flares, and utilization/costs. Results demonstrate a disproportionate burden and potential unmet need in the SLE treatment landscape, particularly for patients treated with high OCS doses who may benefit most from improved care options.
1. Garris C, et al. J Med Econ. 2013;16(5):667-677.

 1Comprehensive SLE-related utilization and costs included and IP or ER claims with an SLE diagnosis, as well as any IP and ER claims for SLE-related comorbidities based on Garris C, Shah M, Farrelly E. Cost Eff Resour Alloc. 2015;13:9.
1Comprehensive SLE-related utilization and costs included and IP or ER claims with an SLE diagnosis, as well as any IP and ER claims for SLE-related comorbidities based on Garris C, Shah M, Farrelly E. Cost Eff Resour Alloc. 2015;13:9. 1Comprehensive SLE-related utilization and costs included and IP or ER claims with an SLE diagnosis, as well as any IP and ER claims for SLE-related comorbidities based on Garris C, Shah M, Farrelly E. Cost Eff Resour Alloc. 2015;13:9.
1Comprehensive SLE-related utilization and costs included and IP or ER claims with an SLE diagnosis, as well as any IP and ER claims for SLE-related comorbidities based on Garris C, Shah M, Farrelly E. Cost Eff Resour Alloc. 2015;13:9.Disclosures: S. Wu, AstraZeneca; A. Perry, None; H. Varker, None; R. Bizier, None; L. Palmer, None; G. Bryant, AstraZeneca, AstraZeneca.

