Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0343–0371) SLE – Treatment Poster I
0367: Comparative Risk of COVID-19 Among Patients with Systemic Lupus Erythematosus According to Immunosuppressant Use
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

April Jorge, MD
Massachusetts General Hospital
Boston, MA, United States
Abstract Poster Presenter(s)
April Jorge1, Baijun Zhou1, yuqing zhang2 and Hyon Choi3, 1Massachusetts General Hospital, Boston, MA, 2Massachusetts General Hospital, Quincy, MA, 3MASSACHUSETTS GENERAL HOSPITAL, Lexington, MA
Background/Purpose: Patients with SLE are at increased risk of COVID-19 and its severe outcomes, in part due to the use of immunosuppressants. We sought to determine the comparative risk of COVID-19 associated with belimumab versus other immunosuppressant use among patients with SLE.
Methods: We conducted a cohort study using TriNetX, a multi-center electronic health record database including 46 health care organizations across the United States. We identified patients aged ≥18 with SLE (≥2 ICD codes ≥2 months and ≤2 years apart) without lupus nephritis (per ICD 9/10 codes) who initiated belimumab, azathioprine, methotrexate, mycophenolate, or rituximab between 1/2019-8/2021. We conducted parallel analyses comparing initiation of belimumab vs. each of the other four immunosuppressants. In each analysis, we used propensity score (PS) overlap weighting to balance covariates, including age, sex, race/ethnicity, geographic region, date of initiation, concomitant medications, comorbidities, SLE severity index (Garrison 2013), and healthcare utilization. The outcome of COVID-19 was identified from ICD codes and laboratory results. We used Cox regression to estimate cumulative incidence and hazard ratios (HRs) of COVID-19 associated with belimumab use vs. azathioprine, methotrexate, mycophenolate, or rituximab at one and two years. We then calculated HRs for COVID-19 associated with belimumab use versus combined immunosuppressants using generalized estimating equations, which addressed correlation within subjects.
Results: Of 71267 patients with SLE, 1885 patients initiated belimumab, 1932 initiated azathioprine, 2196 initiated mycophenolate, 2547 initiated methotrexate, and 697 initiated rituximab during the study period (Table 1). 91.8% were female; mean age was 46.7 years. Glucocorticoids were used by >50%. In each drug-drug comparison, all covariates were balanced with standardized mean differences < 0.01 after PS overlap weighting. In the belimumab vs. azathioprine comparison, the incidence of COVID-19 at one year was 2.5% vs. 4.5%, respectively (HR 0.54 [95% CI 0.33-0.90]) (Table 2). At two years, the incidence was 8.9% vs 10.6% among belimumab and azathioprine users, respectively (HR 0.76 [95% CI 0.50-1.15]). The rates of COVID-19 were numerically lower among belimumab users when compared with mycophenolate, methotrexate, and rituximab, but those individual comparisons did not reach significance. The combined HR for COVID-19 associated with belimumab use versus alternative immunosuppressant use was 0.64 (95% 0.42-0.98) at one year and 0.83 (95% CI 0.59-1.16) at two years (Figure 1).
Conclusion: We found that belimumab use was associated with a lower 1-year risk of COVID-19 compared with alternative SLE immunosuppressants. This effect was moderated at 2 years. As this study period coincided primarily with the alpha variant of SARS-CoV-2, and COVID-19 vaccinations were not widely available until the final months of the study period, we did not assess the impact of newer variants or vaccination on the risk of COVID-19 among belimumab users. However, our findings provide some reassurance that the risk of COVID-19 for SLE patients using belimumab is similar or lower than that of other immunosuppressants.
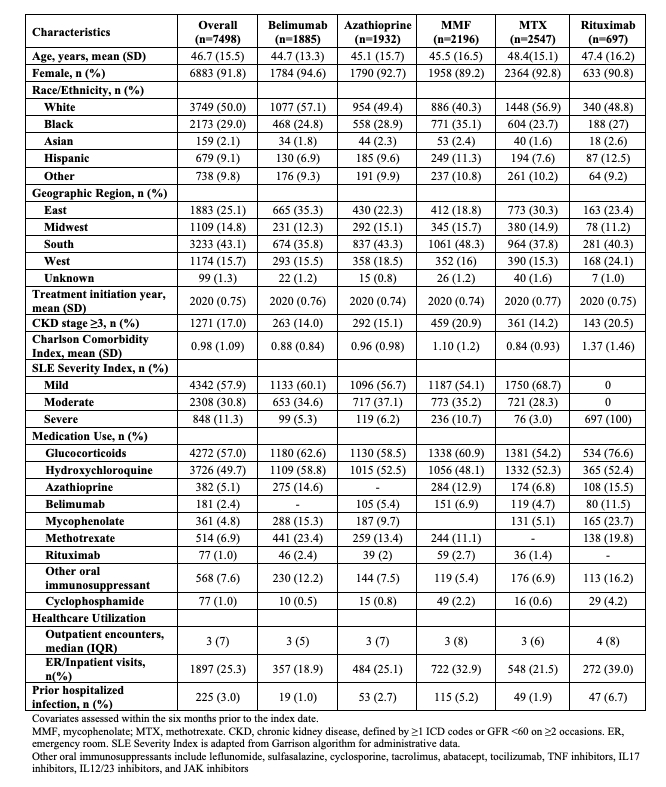 Table 1. Baseline Characteristics of Patients with Systemic Lupus Erythematosus who Initiated an Immunosuppressant Since 2019
Table 1. Baseline Characteristics of Patients with Systemic Lupus Erythematosus who Initiated an Immunosuppressant Since 2019
.jpg) Table 2. Cumulative Incidence of COVID-19 According to Immunosuppressant Treatment for Systemic Lupus Erythematosus
Table 2. Cumulative Incidence of COVID-19 According to Immunosuppressant Treatment for Systemic Lupus Erythematosus
.jpg) Figure 1. Forest Plot of Hazard Ratios Comparing the 1-Year Risk of COVID-19 Associated with Belimumab Versus Other Immunosuppressant Use Among Patients with Systemic Lupus Erythematosus
Figure 1. Forest Plot of Hazard Ratios Comparing the 1-Year Risk of COVID-19 Associated with Belimumab Versus Other Immunosuppressant Use Among Patients with Systemic Lupus Erythematosus
Disclosures: A. Jorge, None; B. Zhou, None; y. zhang, None; H. Choi, Horizon, Allena, LG, Protalix.
Background/Purpose: Patients with SLE are at increased risk of COVID-19 and its severe outcomes, in part due to the use of immunosuppressants. We sought to determine the comparative risk of COVID-19 associated with belimumab versus other immunosuppressant use among patients with SLE.
Methods: We conducted a cohort study using TriNetX, a multi-center electronic health record database including 46 health care organizations across the United States. We identified patients aged ≥18 with SLE (≥2 ICD codes ≥2 months and ≤2 years apart) without lupus nephritis (per ICD 9/10 codes) who initiated belimumab, azathioprine, methotrexate, mycophenolate, or rituximab between 1/2019-8/2021. We conducted parallel analyses comparing initiation of belimumab vs. each of the other four immunosuppressants. In each analysis, we used propensity score (PS) overlap weighting to balance covariates, including age, sex, race/ethnicity, geographic region, date of initiation, concomitant medications, comorbidities, SLE severity index (Garrison 2013), and healthcare utilization. The outcome of COVID-19 was identified from ICD codes and laboratory results. We used Cox regression to estimate cumulative incidence and hazard ratios (HRs) of COVID-19 associated with belimumab use vs. azathioprine, methotrexate, mycophenolate, or rituximab at one and two years. We then calculated HRs for COVID-19 associated with belimumab use versus combined immunosuppressants using generalized estimating equations, which addressed correlation within subjects.
Results: Of 71267 patients with SLE, 1885 patients initiated belimumab, 1932 initiated azathioprine, 2196 initiated mycophenolate, 2547 initiated methotrexate, and 697 initiated rituximab during the study period (Table 1). 91.8% were female; mean age was 46.7 years. Glucocorticoids were used by >50%. In each drug-drug comparison, all covariates were balanced with standardized mean differences < 0.01 after PS overlap weighting. In the belimumab vs. azathioprine comparison, the incidence of COVID-19 at one year was 2.5% vs. 4.5%, respectively (HR 0.54 [95% CI 0.33-0.90]) (Table 2). At two years, the incidence was 8.9% vs 10.6% among belimumab and azathioprine users, respectively (HR 0.76 [95% CI 0.50-1.15]). The rates of COVID-19 were numerically lower among belimumab users when compared with mycophenolate, methotrexate, and rituximab, but those individual comparisons did not reach significance. The combined HR for COVID-19 associated with belimumab use versus alternative immunosuppressant use was 0.64 (95% 0.42-0.98) at one year and 0.83 (95% CI 0.59-1.16) at two years (Figure 1).
Conclusion: We found that belimumab use was associated with a lower 1-year risk of COVID-19 compared with alternative SLE immunosuppressants. This effect was moderated at 2 years. As this study period coincided primarily with the alpha variant of SARS-CoV-2, and COVID-19 vaccinations were not widely available until the final months of the study period, we did not assess the impact of newer variants or vaccination on the risk of COVID-19 among belimumab users. However, our findings provide some reassurance that the risk of COVID-19 for SLE patients using belimumab is similar or lower than that of other immunosuppressants.
 Table 1. Baseline Characteristics of Patients with Systemic Lupus Erythematosus who Initiated an Immunosuppressant Since 2019
Table 1. Baseline Characteristics of Patients with Systemic Lupus Erythematosus who Initiated an Immunosuppressant Since 2019.jpg) Table 2. Cumulative Incidence of COVID-19 According to Immunosuppressant Treatment for Systemic Lupus Erythematosus
Table 2. Cumulative Incidence of COVID-19 According to Immunosuppressant Treatment for Systemic Lupus Erythematosus .jpg) Figure 1. Forest Plot of Hazard Ratios Comparing the 1-Year Risk of COVID-19 Associated with Belimumab Versus Other Immunosuppressant Use Among Patients with Systemic Lupus Erythematosus
Figure 1. Forest Plot of Hazard Ratios Comparing the 1-Year Risk of COVID-19 Associated with Belimumab Versus Other Immunosuppressant Use Among Patients with Systemic Lupus ErythematosusDisclosures: A. Jorge, None; B. Zhou, None; y. zhang, None; H. Choi, Horizon, Allena, LG, Protalix.

