Back
Poster Session C
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1486–1517) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster III
1492: Treatment with Non-steroidal Anti-inflammatory Drugs Is Associated with Retardation of Radiographic Spinal Progression in Patients with Axial Spondyloarthritis: 10-year Results from the GErman SPondyloarthritis Inception Cohort
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Murat Torgutalp, MSc
Berlin School of Public Health - Charité – Universitätsmedizin
Berlin, Germany
Abstract Poster Presenter(s)
Murat Torgutalp1, valeria Rios-Rodriguez2, Ani Dilbaryan3, Fabian Proft4, Mikhail Protopopov5, Maryna Verba6, Judith Rademacher5, Hildrun Haibel1, Joachim Sieper1, Martin Rudwaleit7 and Denis Poddubnyy4, 1Charité - Universitätsmedizin, Berlin, Berlin, Germany, 2Charité-Universitätsmedizin Berlin, Berlin, Germany, 3Department of Internal Medicine, Moscow City Clinical Hospital, Moscow, Russia, 4Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany, 5Charité Universitätsmedizin Berlin, Berlin, Germany, 6Charité-Universitätsmedizin Berlin, corporate member of Freie-Universitat Berlin and Humboldt-Universitat zu Berlin, Department of Gastroenterology, Infectious Diseases and Rheumatology (including Nutrition Medicine), Berlin, Germany, 7University of Bielefeld, Klinikum Bielefeld, Bielefeld; Germany Klinikum Bielefeld and Charité Berlin, Germany, and Gent University, Gent, Belgium
Background/Purpose: There are conflicting data regarding the effect of nonsteroidal anti-inflammatory drugs (NSAID) on radiographic spinal progression in axial spondyloarthritis (axSpA). The analysis of the first 2-year of the GErman SPondyloarthritis Inception Cohort (GESPIC) showed that higher NSAID intake may retard new bone formation in r-axSpA. It remained, however, unclear, whether cyclooxygenase-2 selective inhibitors (COX2i) might have a stronger effect than non-selective (NS) ones and if the effect could be observed also in nr-axSpA. In this study, we aimed to investigate the effect of NSAIDs (COX2i and NS) intake on radiographic spinal progression in patients with r-axSpA and nr-axSpA.
Methods: Based on the availability of at least two sets of spinal radiographs during 10-year follow-up, 243 patients with early axSpA (130 and 113 nr- and r-axSpA, respectively) from GESPIC were included in this analysis. The patients contributed a total of 540 2-year radiographic intervals. Radiographs were scored by 3 trained and calibrated readers according to the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). Final mSASSS was calculated as a mean of 3 readers, and progression was defined as absolute mSASSS change score over 2 years. NSAID type, daily dose, and frequency of intake were recorded at visits. The ASAS index of NSAID intake (0-100) counting both dose and duration of intake was calculated for intervals. The association between NSAID intake (NSAID type and NSAID score) and radiographic spinal progression over 2 years was analyzed using longitudinal generalized estimated equations (GEE).
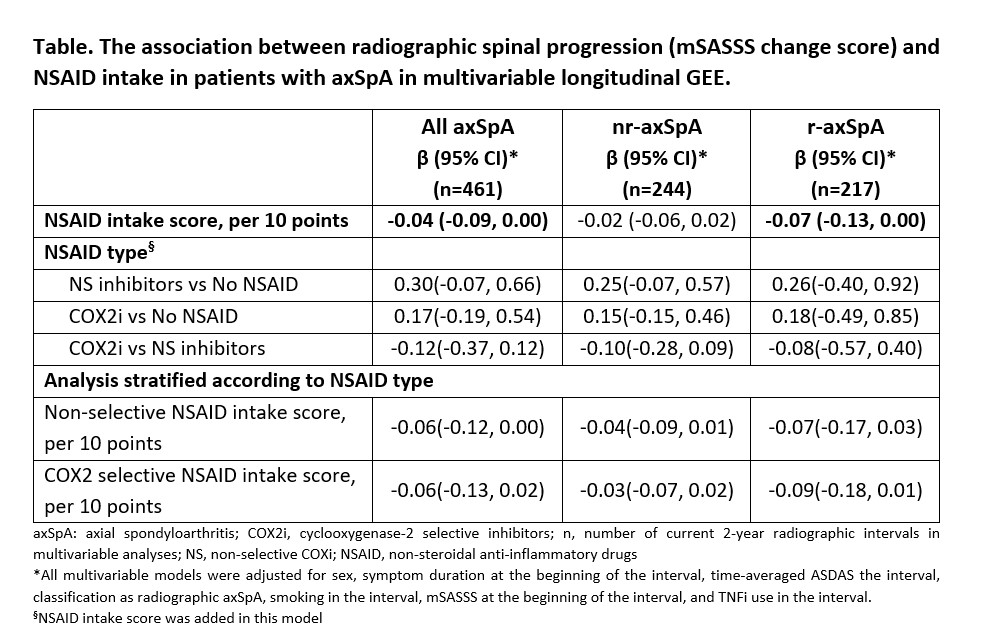
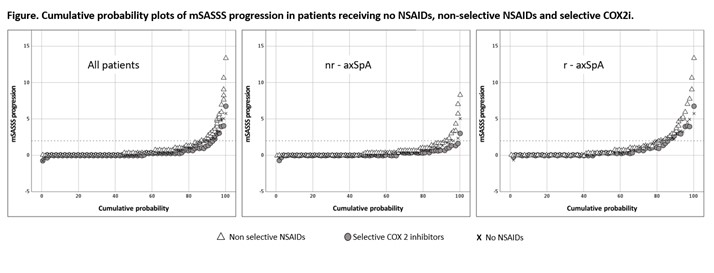
Results: At baseline, 161 (66.3%) patients were treated with NSAIDs. While 289 (53.5%) and 128 (23.7%) 2-year radiographic intervals were covered by NS and COX-2i respectively, 123 (22.8%) intervals were not covered by NSAID. A significant association between higher NSAID intake and retardation of radiographic spinal progression was found in adjusted multivariable longitudinal GEE analysis. This effect was mostly attributable to patients with r-axSpA (Table). mSASSS progression was numerically lower in patients taking COX2i (irrespectively of dose) as compared to patients treated with NS-NSAIDs; in stratified analysis, however, there was no clear dose-dependency (as reflected by NSAID index) in both groups (Figure, Table).
Conclusion: Higher NSAID intake is associated with lower radiographic spinal progression, particularly in r-axSpA patients. COX2i might possess a stronger inhibitory effect on radiographic progression as compared to NS-NSAIDs.
Disclosures: M. Torgutalp, UCB, AbbVie/Abbott; v. Rios-Rodriguez, Falk, AbbVie/Abbott; A. Dilbaryan, None; F. Proft, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Merck/MSD, Novartis, Pfizer, Roche, UCB; M. Protopopov, None; M. Verba, None; J. Rademacher, Novartis, UCB; H. Haibel, Boehringer-Ingelheim, Janssen, Merck/MSD, Novartis, Roche, Sobi, Pfizer, AbbVie/Abbott; J. Sieper, AbbVie/Abbott, Novartis, Eli Lilly, Merck/MSD, UCB, Janssen, Pfizer, Roche; M. Rudwaleit, AbbVie, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Chugai, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB.
Background/Purpose: There are conflicting data regarding the effect of nonsteroidal anti-inflammatory drugs (NSAID) on radiographic spinal progression in axial spondyloarthritis (axSpA). The analysis of the first 2-year of the GErman SPondyloarthritis Inception Cohort (GESPIC) showed that higher NSAID intake may retard new bone formation in r-axSpA. It remained, however, unclear, whether cyclooxygenase-2 selective inhibitors (COX2i) might have a stronger effect than non-selective (NS) ones and if the effect could be observed also in nr-axSpA. In this study, we aimed to investigate the effect of NSAIDs (COX2i and NS) intake on radiographic spinal progression in patients with r-axSpA and nr-axSpA.
Methods: Based on the availability of at least two sets of spinal radiographs during 10-year follow-up, 243 patients with early axSpA (130 and 113 nr- and r-axSpA, respectively) from GESPIC were included in this analysis. The patients contributed a total of 540 2-year radiographic intervals. Radiographs were scored by 3 trained and calibrated readers according to the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). Final mSASSS was calculated as a mean of 3 readers, and progression was defined as absolute mSASSS change score over 2 years. NSAID type, daily dose, and frequency of intake were recorded at visits. The ASAS index of NSAID intake (0-100) counting both dose and duration of intake was calculated for intervals. The association between NSAID intake (NSAID type and NSAID score) and radiographic spinal progression over 2 years was analyzed using longitudinal generalized estimated equations (GEE).
Results: At baseline, 161 (66.3%) patients were treated with NSAIDs. While 289 (53.5%) and 128 (23.7%) 2-year radiographic intervals were covered by NS and COX-2i respectively, 123 (22.8%) intervals were not covered by NSAID. A significant association between higher NSAID intake and retardation of radiographic spinal progression was found in adjusted multivariable longitudinal GEE analysis. This effect was mostly attributable to patients with r-axSpA (Table). mSASSS progression was numerically lower in patients taking COX2i (irrespectively of dose) as compared to patients treated with NS-NSAIDs; in stratified analysis, however, there was no clear dose-dependency (as reflected by NSAID index) in both groups (Figure, Table).
Conclusion: Higher NSAID intake is associated with lower radiographic spinal progression, particularly in r-axSpA patients. COX2i might possess a stronger inhibitory effect on radiographic progression as compared to NS-NSAIDs.


Disclosures: M. Torgutalp, UCB, AbbVie/Abbott; v. Rios-Rodriguez, Falk, AbbVie/Abbott; A. Dilbaryan, None; F. Proft, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Merck/MSD, Novartis, Pfizer, Roche, UCB; M. Protopopov, None; M. Verba, None; J. Rademacher, Novartis, UCB; H. Haibel, Boehringer-Ingelheim, Janssen, Merck/MSD, Novartis, Roche, Sobi, Pfizer, AbbVie/Abbott; J. Sieper, AbbVie/Abbott, Novartis, Eli Lilly, Merck/MSD, UCB, Janssen, Pfizer, Roche; M. Rudwaleit, AbbVie, Bristol-Myers Squibb (BMS), Boehringer-Ingelheim, Chugai, Eli Lilly, Janssen, Novartis, Pfizer, UCB Pharma; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB.

