Back
Poster Session C
Imaging
Session: (1228–1266) Imaging of Rheumatic Diseases Poster
1254: Ultra High‐Frequency Ultrasound of Labial Salivary Glands in Primary Sjögren’s Syndrome: A Promising Tool to Identify Lymphoproliferative Lesions in Unusual Sites
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- GF
Giovanni Fulvio, MD
University of Pisa
Pisa, Pisa, Italy
Abstract Poster Presenter(s)
Giovanni Fulvio1, Rossana Izzetti2, Francesco Ferro3, Gianmaria Governato4, Gaetano La Rocca5, Silvia Fonzetti6, Inmaculada Concepción Navarro García7, Marta Mosca8, Valentina Donati9 and Chiara Baldini1, 1University of Pisa, Pisa, Pisa, Italy, 2Unit of Dentistry and Oral Surgery, Pisa, Toscana, Italy, 3Clinical and Experimental Medicine Department, Azienda Ospedaliero-Universitaria Pisana, Pisa, Pisa, Italy, 4Azienda Ospedaliera Universitaria Pisana, Pisa, Pisa, Italy, 5University of Pisa, Rheumatology Unit, Palermo, Palermo, Italy, 6Azienda Ospedaliera Universitaria Pisana, Pisa, Italy, 7Clinical and Experimental Medicine Department, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy, 8Rheumatology Unit, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy, 9Pathology Unit, University of Pisa, Pisa, Italy
Background/Purpose: Major salivary gland (MSG) ultrasound (US) is a useful tool in Sjögren's syndrome (SS) diagnostic work-up. Interestingly, in a recent case series, specific US findings have been associated with major salivary gland lymphoma (MSG-NHL). Similarly, Ultra High‐frequency Ultrasound (UHFUS) may detect echostructure alterations in labial salivary glands (LSG) with good diagnostic accuracy for SS. Considering that, despite rare, LSG-NHL may be incidentally detected in routine LSG biopsy. Interest has arisen in UHFUS-LSG for the early detection of LSG-NHL. At present, no data are available on LSG lymphoma (LSG-NHL) radiological features.
The aims of the study were to assess:
1) specific US features of MSG-NHL
2) specific UHFUS features of LSG-NHL
3) potential association between US findings and UHFUS findings of NHL.
Methods: From September 2017 to January 2022, consecutive pSS patients with high clinical risk for MSG lymphoma underwent US and histological examination. More recently, in the work up of LSG biopsy, UHFUS was introduced to scan LSG and to locate biopsy sites. MSG-US was performed with an 18 MHz probe, whereas UHFUS-LSG was performed with a 70 MHz probe. OMERACT score was assessed both in MSG and LSG. For suspected lesions additional ultrasonographic features were also analyzed. Patients' clinical, biological, and histological features were collected.
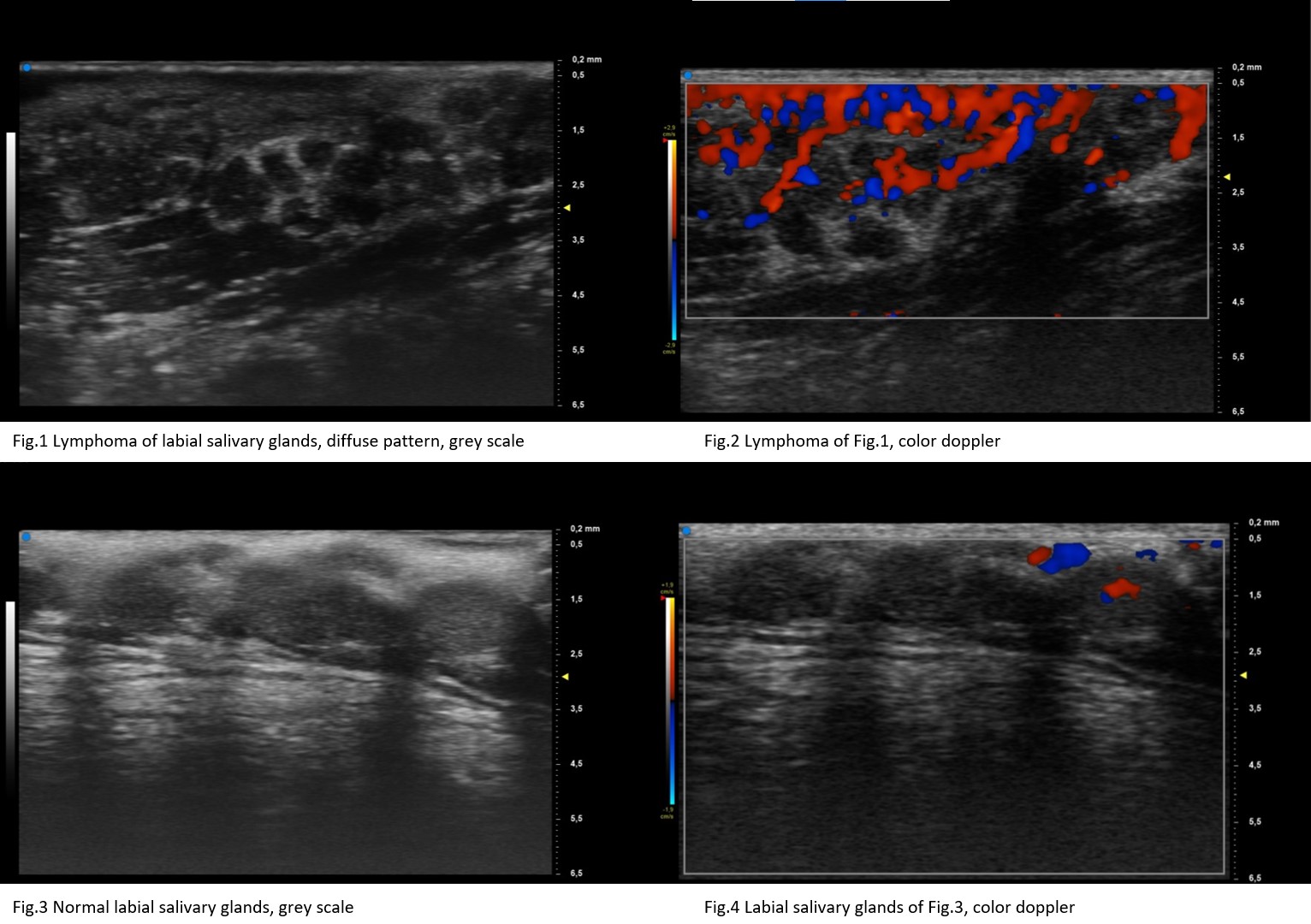
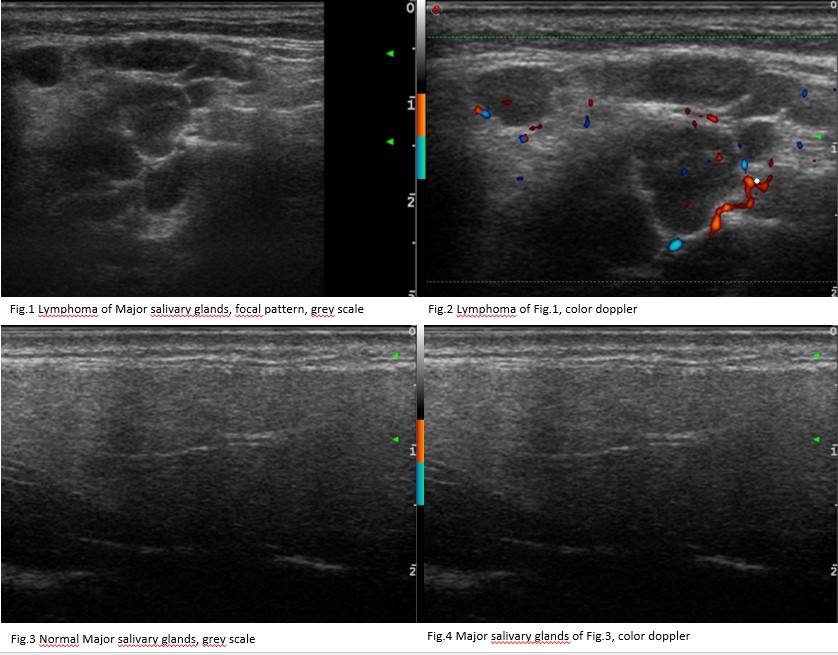
Results: We included 45 consecutive pSS patients with suspected MSG-NHL (MSG group) and 34 consecutive pSS patients undergoing UHFUS-guided LSG biopsy (LSG group). Of suspected MSG-NHL, the diagnosis of NHL was confirmed in 14 patients (17 parotids and 1 submandibular gland) whereas out of 34 pSS patients undergoing UHFUS-guided LSG biopsy (LSG group), 5 atypical inflammatory infiltrate or LSG-NHL were detected (2/5 atypical inflammatory infiltrate and 3/5 labial NHLs, respectively). In MSG group, 16 MSG-NHL glands presented an OMERACT grade 3 while the other two MSG-NHL glands had a score 2. MSG-NHL appeared as very hypoechoic lesions with posterior enhancement, hyperechoic strands or septa and a high peri/intralesional doppler. In LSG group, all 5 atypical inflammatory infiltrate/LSG-NHL had OMERACT score 3, whereas the highest OMERACT score was detected only in 10/29 no-LSG-NHL patients (p=0.05). Of 5 typical inflammatory infiltrate/LSG-NHL patients, 4 presented a diffuse change, while the other patient presented a focal lesion. Atypical inflammatory infiltrate/LSG-NHL appeared as very hypoechoic lesion with high perilesional doppler.
Conclusion: LSG-NHL showed US features similar to MSG-NHL. Patients with an OMERACT score 3 in both their MSG and LSG, presenting very hypoechoic areas and high perilesional doppler, deserve a careful screening for lymphoma.
Disclosures: G. Fulvio, None; R. Izzetti, None; F. Ferro, None; G. Governato, None; G. La Rocca, None; S. Fonzetti, None; I. Navarro García, None; M. Mosca, None; V. Donati, None; C. Baldini, None.
Background/Purpose: Major salivary gland (MSG) ultrasound (US) is a useful tool in Sjögren's syndrome (SS) diagnostic work-up. Interestingly, in a recent case series, specific US findings have been associated with major salivary gland lymphoma (MSG-NHL). Similarly, Ultra High‐frequency Ultrasound (UHFUS) may detect echostructure alterations in labial salivary glands (LSG) with good diagnostic accuracy for SS. Considering that, despite rare, LSG-NHL may be incidentally detected in routine LSG biopsy. Interest has arisen in UHFUS-LSG for the early detection of LSG-NHL. At present, no data are available on LSG lymphoma (LSG-NHL) radiological features.
The aims of the study were to assess:
1) specific US features of MSG-NHL
2) specific UHFUS features of LSG-NHL
3) potential association between US findings and UHFUS findings of NHL.
Methods: From September 2017 to January 2022, consecutive pSS patients with high clinical risk for MSG lymphoma underwent US and histological examination. More recently, in the work up of LSG biopsy, UHFUS was introduced to scan LSG and to locate biopsy sites. MSG-US was performed with an 18 MHz probe, whereas UHFUS-LSG was performed with a 70 MHz probe. OMERACT score was assessed both in MSG and LSG. For suspected lesions additional ultrasonographic features were also analyzed. Patients' clinical, biological, and histological features were collected.
Results: We included 45 consecutive pSS patients with suspected MSG-NHL (MSG group) and 34 consecutive pSS patients undergoing UHFUS-guided LSG biopsy (LSG group). Of suspected MSG-NHL, the diagnosis of NHL was confirmed in 14 patients (17 parotids and 1 submandibular gland) whereas out of 34 pSS patients undergoing UHFUS-guided LSG biopsy (LSG group), 5 atypical inflammatory infiltrate or LSG-NHL were detected (2/5 atypical inflammatory infiltrate and 3/5 labial NHLs, respectively). In MSG group, 16 MSG-NHL glands presented an OMERACT grade 3 while the other two MSG-NHL glands had a score 2. MSG-NHL appeared as very hypoechoic lesions with posterior enhancement, hyperechoic strands or septa and a high peri/intralesional doppler. In LSG group, all 5 atypical inflammatory infiltrate/LSG-NHL had OMERACT score 3, whereas the highest OMERACT score was detected only in 10/29 no-LSG-NHL patients (p=0.05). Of 5 typical inflammatory infiltrate/LSG-NHL patients, 4 presented a diffuse change, while the other patient presented a focal lesion. Atypical inflammatory infiltrate/LSG-NHL appeared as very hypoechoic lesion with high perilesional doppler.
Conclusion: LSG-NHL showed US features similar to MSG-NHL. Patients with an OMERACT score 3 in both their MSG and LSG, presenting very hypoechoic areas and high perilesional doppler, deserve a careful screening for lymphoma.


Disclosures: G. Fulvio, None; R. Izzetti, None; F. Ferro, None; G. Governato, None; G. La Rocca, None; S. Fonzetti, None; I. Navarro García, None; M. Mosca, None; V. Donati, None; C. Baldini, None.

