Back
Poster Session C
Systemic lupus erythematosus (SLE)
Session: (1440–1485) SLE – Diagnosis, Manifestations, and Outcomes Poster II: Manifestations
1448: Utility of Digital Signals and Patient Reported Data Gathered in a Decentralized Study to Predict SLE Patient-Reported Flares
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- EJ
Eldon Jupe, PhD
Progentec Diagnostics, Inc.
Oklahoma City, OK, United States
Abstract Poster Presenter(s)
Eldon Jupe1, Gerald Lushington2, Mohan Purushothaman1, Fabricio Pautasso1, Georg Armstrong1, Arif Sorathia1, Jessica Crawley1, Vijay Nadipelli3, Bernie Rubin4, Ryan Newhardt1, Melissa Munroe1 and Brett Adelman1, 1Progentec Diagnostics, Inc., Oklahoma City, OK, 2Progentec Diagnostics, Inc., Oklahoma City, OR, 3GlaxoSmithKline, Philadelphia, PA, 4GlaxoSmithKline, US Medical Affairs and Immuno-inflammation, Durham, NC
Background/Purpose: Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease characterized by varied immune dysregulation. SLE patients often experience episodic flares, leading to organ damage and poor quality of life. A fully decentralized Mobile Study to Measure and Predict Lupus Disease Activity Using Digital Signals (OASIS) was conducted to explore the utility of digital signals and patient reported data in predicting impending SLE patient-reported flares.
Methods: We recruited study participants from Progentec's LupusCorner community of ~120,000 patients, using digital marketing linked to online eligibility screening procedures. Adult, non-pregnant, patients with a self-reported diagnosis of SLE were appropriately consented and provided a mobile application (security/privacy protocol compliant) to record patient profile (PP) information (demographics, symptoms, and medication use) and quality of life (QOL) metrics, including the SLEQOL instrument. Patients who agreed to fully participate in the six-month study were provided a smartwatch to record physical activity (PA) parameters, heart rate, and sleep duration/depth. PP, QOL, and PA data were examined in aggregate via linear regression for their capacity to predict emergent patient reported SLE 'flare' scores. The resulting model was derived from feature selection using the ClassifierAttributeEval method (E. Frank et al. The WEKA Workbench. Morgan Kaufmann. 2016) and validated using 5-fold cross-validation.
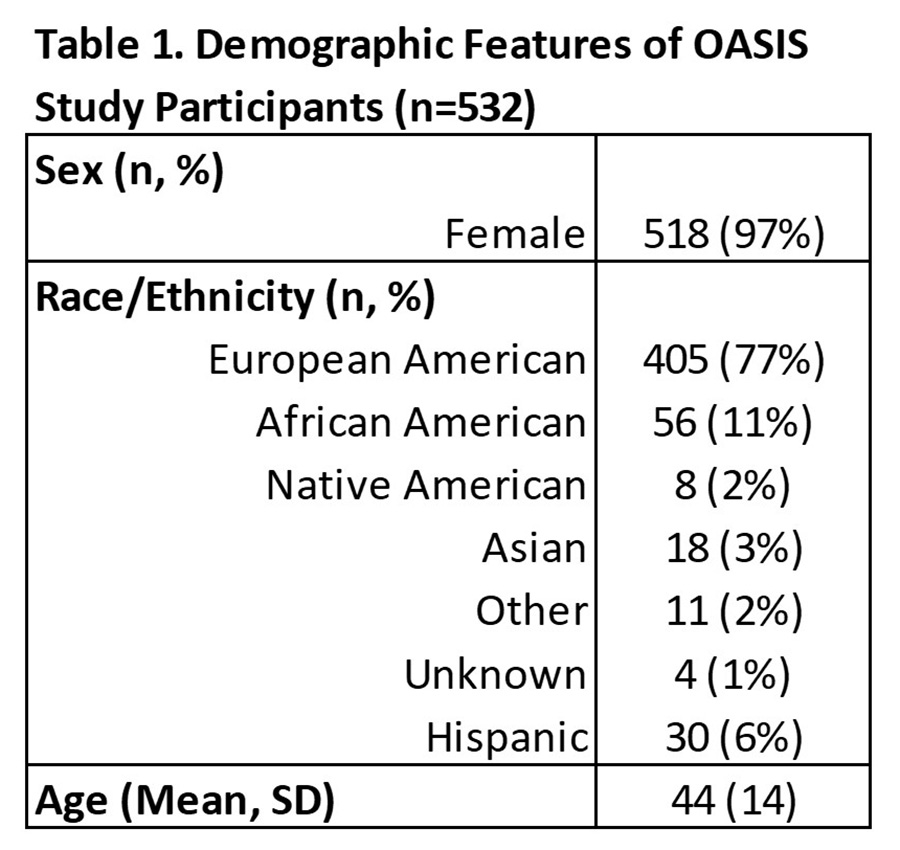
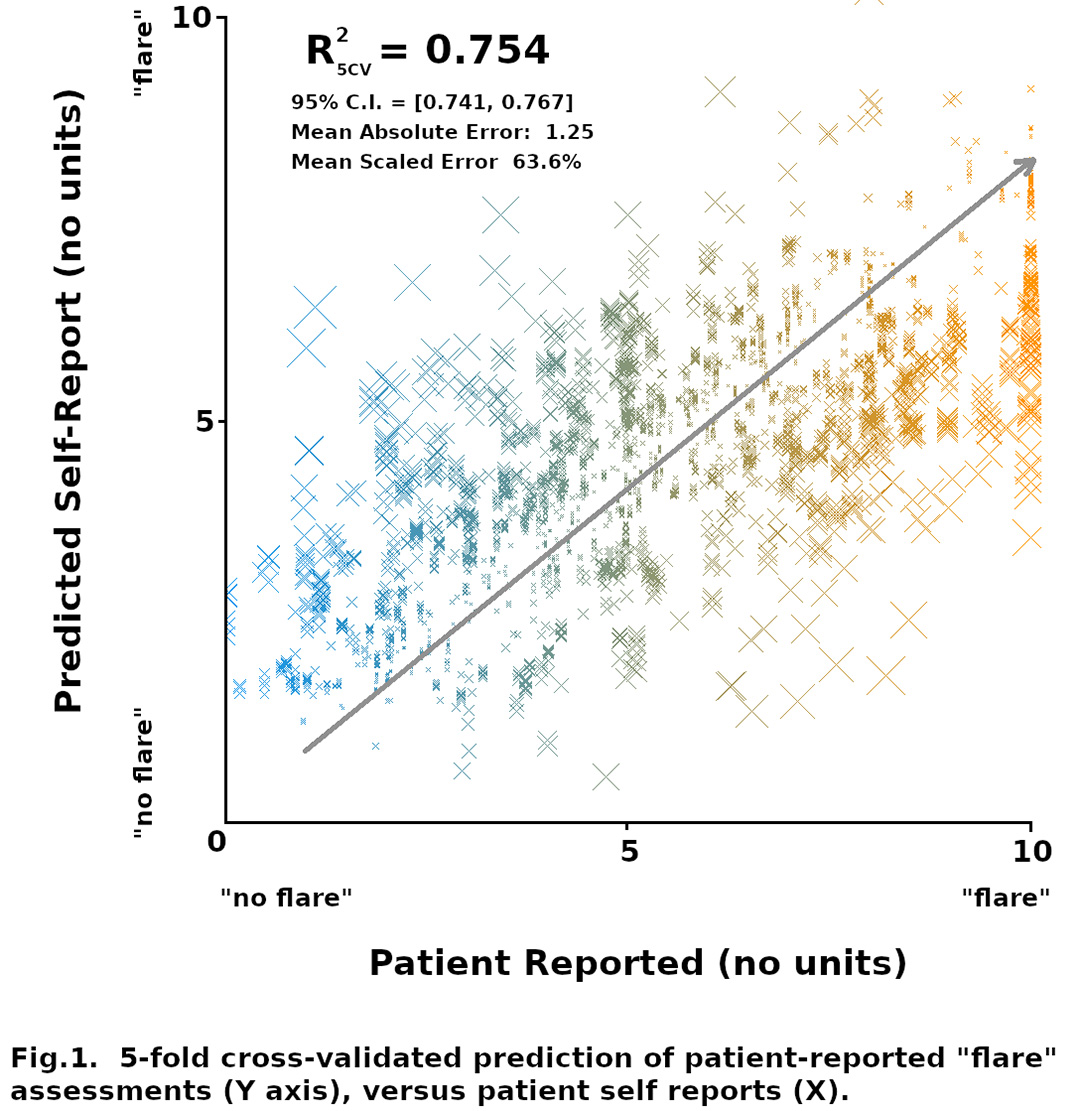
Results: A total of 532 participants (self-reported SLE patients) consented and actively participated in the study; the majority were female and of European American descent, with an average age of 44 years (Table 1). Most participants (88%) reported being treated by a rheumatologist. A total of 144 participants (27%) consented to wearing the smartwatch device. These data, in conjunction with PP and QOL data, were used to derive a 94-feature regression model (Fig. 1) whose correlation coefficient (R2 = 0.75 ± 0.13; 95% CI) implies a significant but approximate (mean relative error = 63.2%) capacity to predict emergent self-reported SLE 'flare' scores. Descriptors with the high feature selection loadings included quantified PA values for moderate and intense patient physical activity, plus self-reported QOL, physical discomfort including epidermal, joint, oral pain, and other concerns serious enough to require medical attention in days prior to self-reported flare. Time-invariant PP terms generally exhibited low self-reported flare sensitivity, but several PP terms (relating to patient responsibilities, such as childcare) had substantial loadings, which implies that demographic profiles influence which time-variant metrics most affect 'flare' activity for different patient subgroups.
Conclusion: Regular profiling of patient self-reported well-being and biometric activity may offer promising screening potential to identify patients warranting further clinical assessment. Our study findings indicate the need to integrate additional data from medical records to further refine and validate these predictive models.
Disclosures: E. Jupe, Progentec Diagnostics, Inc.; G. Lushington, Progentec Diagnostics, Inc.; M. Purushothaman, Progentec Diagnostics, Inc.; F. Pautasso, Progentec Diagnostics, Inc.; G. Armstrong, Progentec Diagnostics, Inc.; A. Sorathia, Progentec Diagnostics, Inc.; J. Crawley, Progentec Diagnostics, Inc.; V. Nadipelli, GlaxoSmithKlein(GSK); B. Rubin, GlaxoSmithKline (GSK); R. Newhardt, None; M. Munroe, Progentec Diagnostics, Inc.; B. Adelman, Progentec Diagnostics, Inc..
Background/Purpose: Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease characterized by varied immune dysregulation. SLE patients often experience episodic flares, leading to organ damage and poor quality of life. A fully decentralized Mobile Study to Measure and Predict Lupus Disease Activity Using Digital Signals (OASIS) was conducted to explore the utility of digital signals and patient reported data in predicting impending SLE patient-reported flares.
Methods: We recruited study participants from Progentec's LupusCorner community of ~120,000 patients, using digital marketing linked to online eligibility screening procedures. Adult, non-pregnant, patients with a self-reported diagnosis of SLE were appropriately consented and provided a mobile application (security/privacy protocol compliant) to record patient profile (PP) information (demographics, symptoms, and medication use) and quality of life (QOL) metrics, including the SLEQOL instrument. Patients who agreed to fully participate in the six-month study were provided a smartwatch to record physical activity (PA) parameters, heart rate, and sleep duration/depth. PP, QOL, and PA data were examined in aggregate via linear regression for their capacity to predict emergent patient reported SLE 'flare' scores. The resulting model was derived from feature selection using the ClassifierAttributeEval method (E. Frank et al. The WEKA Workbench. Morgan Kaufmann. 2016) and validated using 5-fold cross-validation.
Results: A total of 532 participants (self-reported SLE patients) consented and actively participated in the study; the majority were female and of European American descent, with an average age of 44 years (Table 1). Most participants (88%) reported being treated by a rheumatologist. A total of 144 participants (27%) consented to wearing the smartwatch device. These data, in conjunction with PP and QOL data, were used to derive a 94-feature regression model (Fig. 1) whose correlation coefficient (R2 = 0.75 ± 0.13; 95% CI) implies a significant but approximate (mean relative error = 63.2%) capacity to predict emergent self-reported SLE 'flare' scores. Descriptors with the high feature selection loadings included quantified PA values for moderate and intense patient physical activity, plus self-reported QOL, physical discomfort including epidermal, joint, oral pain, and other concerns serious enough to require medical attention in days prior to self-reported flare. Time-invariant PP terms generally exhibited low self-reported flare sensitivity, but several PP terms (relating to patient responsibilities, such as childcare) had substantial loadings, which implies that demographic profiles influence which time-variant metrics most affect 'flare' activity for different patient subgroups.
Conclusion: Regular profiling of patient self-reported well-being and biometric activity may offer promising screening potential to identify patients warranting further clinical assessment. Our study findings indicate the need to integrate additional data from medical records to further refine and validate these predictive models.


Disclosures: E. Jupe, Progentec Diagnostics, Inc.; G. Lushington, Progentec Diagnostics, Inc.; M. Purushothaman, Progentec Diagnostics, Inc.; F. Pautasso, Progentec Diagnostics, Inc.; G. Armstrong, Progentec Diagnostics, Inc.; A. Sorathia, Progentec Diagnostics, Inc.; J. Crawley, Progentec Diagnostics, Inc.; V. Nadipelli, GlaxoSmithKlein(GSK); B. Rubin, GlaxoSmithKline (GSK); R. Newhardt, None; M. Munroe, Progentec Diagnostics, Inc.; B. Adelman, Progentec Diagnostics, Inc..

