Back
Poster Session C
Imaging
Session: (1228–1266) Imaging of Rheumatic Diseases Poster
1242: Validation of a Novel Ultrasound Scoring System for the Evaluation of Pediatric Knee Arthritis
Sunday, November 13, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.png)
Patricia Vega-Fernandez, MD, RhMSUS, MSc
Cincinnati Children's Hospital Medical Center
Cincinnati, OH, United States
Abstract Poster Presenter(s)
Patricia Vega-Fernandez1, Theresa Hennard1, Kelly Rogers1, Mekibib Altaye2, Sherry Thornton2, Alyssa Sproles2, Amy Cassedy3, Arthur Meyers1 and Tracy Ting1, 1Cincinnati Children's Hospital Medical Center, Cincinnati, OH, 2Cincinnati Children's Hospital, Cincinnati, OH, 3Cincinnati Children's hospital medical center, Cleveland, OH
Background/Purpose: Juvenile Idiopathic Arthritis (JIA) is the most common chronic rheumatic disease in children. Currently, clinical evaluation of arthritis is subjective and provider dependent. Musculoskeletal ultrasound (MSUS) is an objective imaging technique that can be used to assess joint inflammation. This study aims to validate a novel pediatric-specific MSUS scoring system[1] for the assessment of knee arthritis.
Methods: Children with a diagnosis of JIA who received a MSUS of the knee(s) were eligible for this study. Clinical data collected included knee physical examination (PE) findings, and physician- and patient- reported outcomes. A comprehensive knee MSUS examination, including B-mode and Power Doppler (PD) mode, was performed on all participants by an American College of Rheumatology MSUS certified pediatric rheumatologist. MSUS images were scored by pediatric MSUS experts, who were blinded to clinical and imaging information, as per recently published semiquantitative MSUS scoring system (0-normal to 3-severe). For the current report, a composite MSUS score was calculated by adding the abnormal B-mode MSUS scores (score 2 or 3) or the abnormal PD MSUS scores (score 1, 2, or 3) from the 3 possible views of MSUS-knee (MSUS composite score range: B-mode 0-6, PD-mode 0-9).
A subset of participants received an MRI with and without contrast of the knee immediately after MSUS performance. MRI of the knee was scored for presence and severity of synovial thickening (ST) and joint effusion (JE) as per MRI Scoring Systems. Synovial fluid (SF) was collected from a subset of participants that received a clinically indicated knee joint injection following MSUS performance. The following biomarkers were measured to evaluate the association of MSUS with local biomarkers of inflammation: TNFa, IL-6, IL-10, MCP-1, ANG-2, MMP-8, IFNg, IL-17a, IL-2Ra, MIG, VEGF, IL-18 by Luminex, and S100A8/A9 by ELISA. Spearman's Correlations were used to calculate the associations between variables.
Results: Sixty-two children with JIA (mean age of 12.5 years) contributed 70 knee MSUS. At the time of MSUS collection, 50% of the knees had arthritis by PE. B-mode MSUS composite score moderately correlated with PE findings of knee arthritis (Table 1). Twenty-three knee MRIs were obtained. B-mode MSUS composite score strongly correlated with MRI determined ST and JE (Table 2). SF was collected from 12 participants following MSUS examination. Table 3 indicates the concentration of SF biomarkers. Preliminary analysis suggests that SF levels of IL-10 (0.70, p-0.011), IFNg (0.72, p-0.008), and MIG (0.59, p-0.04) have a significant association with the B-mode MSUS composite score.
Conclusion: The moderate correlation of MSUS synovitis with knee arthritis by PE and the strong correlation of MSUS synovitis with contrast-enhanced MRI, suggest that MSUS provides an objective bedside assessment of knee arthritis. MSUS has the potential to effectively inform JIA medical decision making real-time. Further work is needed to clarify the association of MSUS with SF biomarkers of inflammation.
1. Ting, T.V., et al., Novel Ultrasound Image Acquisition Protocol and Scoring System for the Pediatric Knee.Arthritis Care Res, 2019. 71: 977-85.
.jpg) Table 1. Association between clinical exam* and MSUS findings of knee synovitis. *Clinical knee synovitis was determined by expert pediatric rheumatologist clinician as the presence of knee joint swelling due to inflammation or in the absence of swelling then loss of motion with either pain on motion and/or tenderness.
Table 1. Association between clinical exam* and MSUS findings of knee synovitis. *Clinical knee synovitis was determined by expert pediatric rheumatologist clinician as the presence of knee joint swelling due to inflammation or in the absence of swelling then loss of motion with either pain on motion and/or tenderness.
Strength of the correlation calculated using Spearman correlation as follows: very weak: 0.0-0.19, weak: 0.2-0.39, moderate 0.4-0.59, strong 0.6-0.79, very strong 0.8-1.0. An alpha of p < 0.05 was considered significant. All analysis was conducted using SAS 9.4©.
.jpg) Table 2. Associations between B-mode composite score (0-6) and MRI scores. aMRI ST Binary: presence or absence of synovial thickening (ST) on MRI.
Table 2. Associations between B-mode composite score (0-6) and MRI scores. aMRI ST Binary: presence or absence of synovial thickening (ST) on MRI.
bMRI ST 6 areas: scoring of ST as per Juvenile Arthritis MRI Scoring System (JAMRIS), score range (0-12)*
cMRI composite ST and JE: MRI ST 6 areas plus quantification of joint (JE) as per the International Prophylaxis Study Group (IPSG), score range (0-15)1
Strength of the correlation calculated using Spearman correlation as follows: very weak: 0.0-0.19, weak: 0.2-0.39, moderate 0.4-0.59, strong 0.6-0.79, very strong 0.8-1.0. An alpha of p < 0.05 was considered significant. All analysis was conducted using SAS 9.4©.
1Hemke, R., et al., Magnetic Resonance Imaging (MRI) of the Knee as an Outcome Measure in Juvenile Idiopathic Arthritis: An OMERACT Reliability Study on MRI Scales. J Rheumatol, 2017. 44(8): p. 1224-1230.
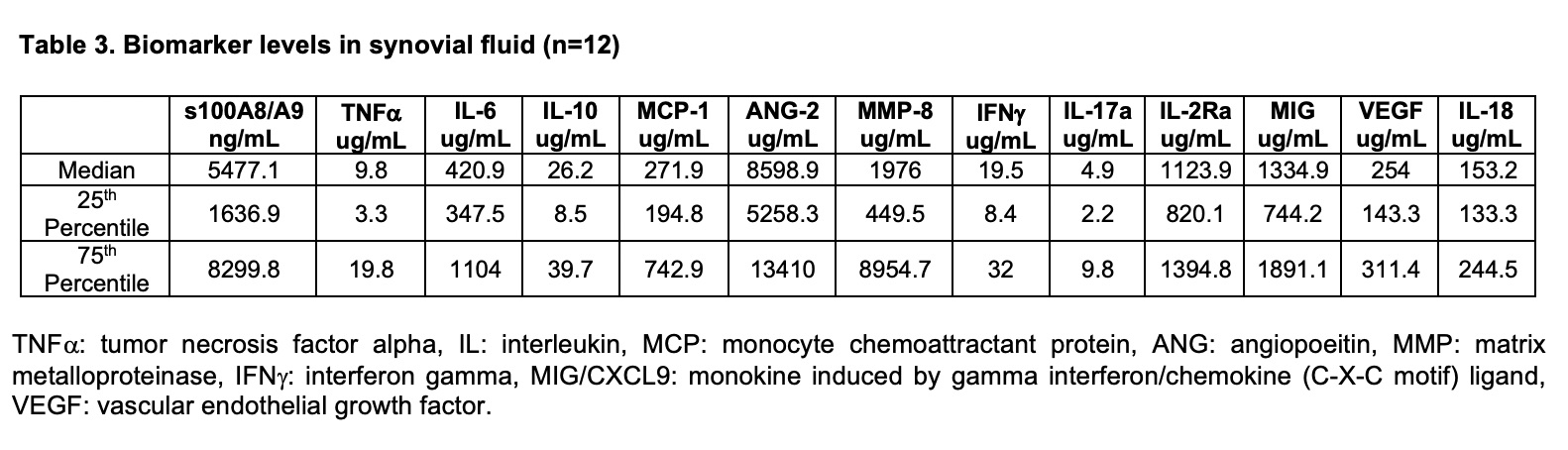 Table 3. Biomarker levels in synovial fluid (n=12). TNF alpha: tumor necrosis factor alpha, IL: interleukin, MCP: monocyte chemoattractant protein, ANG: angiopoeitin, MMP: matrix metalloproteinase, IFN gamma: interferon gamma, MIG/CXCL9: monokine induced by gamma interferon/chemokine (C-X-C motif) ligand, VEGF: vascular endothelial growth factor.
Table 3. Biomarker levels in synovial fluid (n=12). TNF alpha: tumor necrosis factor alpha, IL: interleukin, MCP: monocyte chemoattractant protein, ANG: angiopoeitin, MMP: matrix metalloproteinase, IFN gamma: interferon gamma, MIG/CXCL9: monokine induced by gamma interferon/chemokine (C-X-C motif) ligand, VEGF: vascular endothelial growth factor.
Disclosures: P. Vega-Fernandez, None; T. Hennard, None; K. Rogers, None; M. Altaye, None; S. Thornton, None; A. Sproles, None; A. Cassedy, None; A. Meyers, Amirsys/Elsevier, Pfizer; T. Ting, None.
Background/Purpose: Juvenile Idiopathic Arthritis (JIA) is the most common chronic rheumatic disease in children. Currently, clinical evaluation of arthritis is subjective and provider dependent. Musculoskeletal ultrasound (MSUS) is an objective imaging technique that can be used to assess joint inflammation. This study aims to validate a novel pediatric-specific MSUS scoring system[1] for the assessment of knee arthritis.
Methods: Children with a diagnosis of JIA who received a MSUS of the knee(s) were eligible for this study. Clinical data collected included knee physical examination (PE) findings, and physician- and patient- reported outcomes. A comprehensive knee MSUS examination, including B-mode and Power Doppler (PD) mode, was performed on all participants by an American College of Rheumatology MSUS certified pediatric rheumatologist. MSUS images were scored by pediatric MSUS experts, who were blinded to clinical and imaging information, as per recently published semiquantitative MSUS scoring system (0-normal to 3-severe). For the current report, a composite MSUS score was calculated by adding the abnormal B-mode MSUS scores (score 2 or 3) or the abnormal PD MSUS scores (score 1, 2, or 3) from the 3 possible views of MSUS-knee (MSUS composite score range: B-mode 0-6, PD-mode 0-9).
A subset of participants received an MRI with and without contrast of the knee immediately after MSUS performance. MRI of the knee was scored for presence and severity of synovial thickening (ST) and joint effusion (JE) as per MRI Scoring Systems. Synovial fluid (SF) was collected from a subset of participants that received a clinically indicated knee joint injection following MSUS performance. The following biomarkers were measured to evaluate the association of MSUS with local biomarkers of inflammation: TNFa, IL-6, IL-10, MCP-1, ANG-2, MMP-8, IFNg, IL-17a, IL-2Ra, MIG, VEGF, IL-18 by Luminex, and S100A8/A9 by ELISA. Spearman's Correlations were used to calculate the associations between variables.
Results: Sixty-two children with JIA (mean age of 12.5 years) contributed 70 knee MSUS. At the time of MSUS collection, 50% of the knees had arthritis by PE. B-mode MSUS composite score moderately correlated with PE findings of knee arthritis (Table 1). Twenty-three knee MRIs were obtained. B-mode MSUS composite score strongly correlated with MRI determined ST and JE (Table 2). SF was collected from 12 participants following MSUS examination. Table 3 indicates the concentration of SF biomarkers. Preliminary analysis suggests that SF levels of IL-10 (0.70, p-0.011), IFNg (0.72, p-0.008), and MIG (0.59, p-0.04) have a significant association with the B-mode MSUS composite score.
Conclusion: The moderate correlation of MSUS synovitis with knee arthritis by PE and the strong correlation of MSUS synovitis with contrast-enhanced MRI, suggest that MSUS provides an objective bedside assessment of knee arthritis. MSUS has the potential to effectively inform JIA medical decision making real-time. Further work is needed to clarify the association of MSUS with SF biomarkers of inflammation.
1. Ting, T.V., et al., Novel Ultrasound Image Acquisition Protocol and Scoring System for the Pediatric Knee.Arthritis Care Res, 2019. 71: 977-85.
.jpg) Table 1. Association between clinical exam* and MSUS findings of knee synovitis. *Clinical knee synovitis was determined by expert pediatric rheumatologist clinician as the presence of knee joint swelling due to inflammation or in the absence of swelling then loss of motion with either pain on motion and/or tenderness.
Table 1. Association between clinical exam* and MSUS findings of knee synovitis. *Clinical knee synovitis was determined by expert pediatric rheumatologist clinician as the presence of knee joint swelling due to inflammation or in the absence of swelling then loss of motion with either pain on motion and/or tenderness.Strength of the correlation calculated using Spearman correlation as follows: very weak: 0.0-0.19, weak: 0.2-0.39, moderate 0.4-0.59, strong 0.6-0.79, very strong 0.8-1.0. An alpha of p < 0.05 was considered significant. All analysis was conducted using SAS 9.4©.
.jpg) Table 2. Associations between B-mode composite score (0-6) and MRI scores. aMRI ST Binary: presence or absence of synovial thickening (ST) on MRI.
Table 2. Associations between B-mode composite score (0-6) and MRI scores. aMRI ST Binary: presence or absence of synovial thickening (ST) on MRI. bMRI ST 6 areas: scoring of ST as per Juvenile Arthritis MRI Scoring System (JAMRIS), score range (0-12)*
cMRI composite ST and JE: MRI ST 6 areas plus quantification of joint (JE) as per the International Prophylaxis Study Group (IPSG), score range (0-15)1
Strength of the correlation calculated using Spearman correlation as follows: very weak: 0.0-0.19, weak: 0.2-0.39, moderate 0.4-0.59, strong 0.6-0.79, very strong 0.8-1.0. An alpha of p < 0.05 was considered significant. All analysis was conducted using SAS 9.4©.
1Hemke, R., et al., Magnetic Resonance Imaging (MRI) of the Knee as an Outcome Measure in Juvenile Idiopathic Arthritis: An OMERACT Reliability Study on MRI Scales. J Rheumatol, 2017. 44(8): p. 1224-1230.
 Table 3. Biomarker levels in synovial fluid (n=12). TNF alpha: tumor necrosis factor alpha, IL: interleukin, MCP: monocyte chemoattractant protein, ANG: angiopoeitin, MMP: matrix metalloproteinase, IFN gamma: interferon gamma, MIG/CXCL9: monokine induced by gamma interferon/chemokine (C-X-C motif) ligand, VEGF: vascular endothelial growth factor.
Table 3. Biomarker levels in synovial fluid (n=12). TNF alpha: tumor necrosis factor alpha, IL: interleukin, MCP: monocyte chemoattractant protein, ANG: angiopoeitin, MMP: matrix metalloproteinase, IFN gamma: interferon gamma, MIG/CXCL9: monokine induced by gamma interferon/chemokine (C-X-C motif) ligand, VEGF: vascular endothelial growth factor.Disclosures: P. Vega-Fernandez, None; T. Hennard, None; K. Rogers, None; M. Altaye, None; S. Thornton, None; A. Sproles, None; A. Cassedy, None; A. Meyers, Amirsys/Elsevier, Pfizer; T. Ting, None.

