Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0297: Effect of Upadacitinib, Adalimumab, and Placebo on Residual Pain Among Patients with Rheumatoid Arthritis Whose Inflammation Was Attenuated After Three and Six Months of Treatment
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- LB
Louis Bessette, MD, MSc
Centre de l'Ostéoporose et de Rhumatologie de Québec
Quebec, QC, Canada
Abstract Poster Presenter(s)
Louis Bessette1, Adriana Kakehasi2, Neil Basu3, David A. Walsh4, Andra Balanescu5, Clifton Bingham6, Andrew Garrison7, Ivan Lagunes8, sander strengholt9, Ralph Lippe10 and Maxime Dougados11, 1Centre de l'Ostoporose et de Rhumatologie de Québec, Québec, QC, Canada, 2Federal University of Minas Gerais, Hospital das Clínicas, Belo Horizonte, Brazil, 3University of Glasgow, Glasgow, Scotland, United Kingdom, 4University of Nottingham, Nottingham, United Kingdom, 5University of Medicine and Pharmacy Carol Davila, Bucharest, Romania, 6Johns Hopkins University, Baltimore, MD, 7AbbVie, Inc., North Chicago, IL, 8AbbVie, Inc., Mettawa, IL, 9AbbVie, Inc., Abcoude, Netherlands, 10AbbVie, Inc, Wiesbaden, Germany, 11Université de Paris, Paris, France
Background/Purpose: Residual pain remains a challenge that prevents patients (pts) with RA from achieving a better quality of life. Current RA treatments have demonstrated efficacy in reducing pain. Janus kinase inhibitor upadacitinib (UPA)+background MTX demonstrated higher improvements in pain vs adalimumab (ADA)+MTX and placebo (PBO)+MTX in the SELECT-COMPARE study.1 The objective of this post-hoc analysis was to describe levels of pain reduction and residual pain after treatment with UPA+MTX vs ADA+MTX and PBO+MTX in pts with RA with and without attenuation of inflammation at weeks 12 and 26.
Methods: Pts with active RA receiving background MTX were randomized to UPA 15 mg once daily, ADA 40 mg every other week, or PBO. Subgroup assessment between pts with attenuation of inflammation (defined as swollen joint count based on 66 joints (SJC66) of 0 and CRP < 6 mg/L) vs remaining inflammation at week 12 and week 26 was conducted. Pain was measured by a visual analogue scale (0 ["no pain"] to 100 mm ["most severe pain"]). Mean change from baseline in pain at week 12 and week 26 as well as ≥30%, ≥50%, or ≥70% reduction from baseline to week 12 and week 26 in pain were assessed. Observed case data are reported in full analysis set, including all randomized pts who had received at least one dose of study drug. For change from baseline analyses, between group least squares mean, 95% confidence interval, and nominal P value were based on analysis of covariance model with treatment as a fixed factor and baseline value as a covariate. For binary endpoint analyses, nominal P values were calculated by the chi-square test.
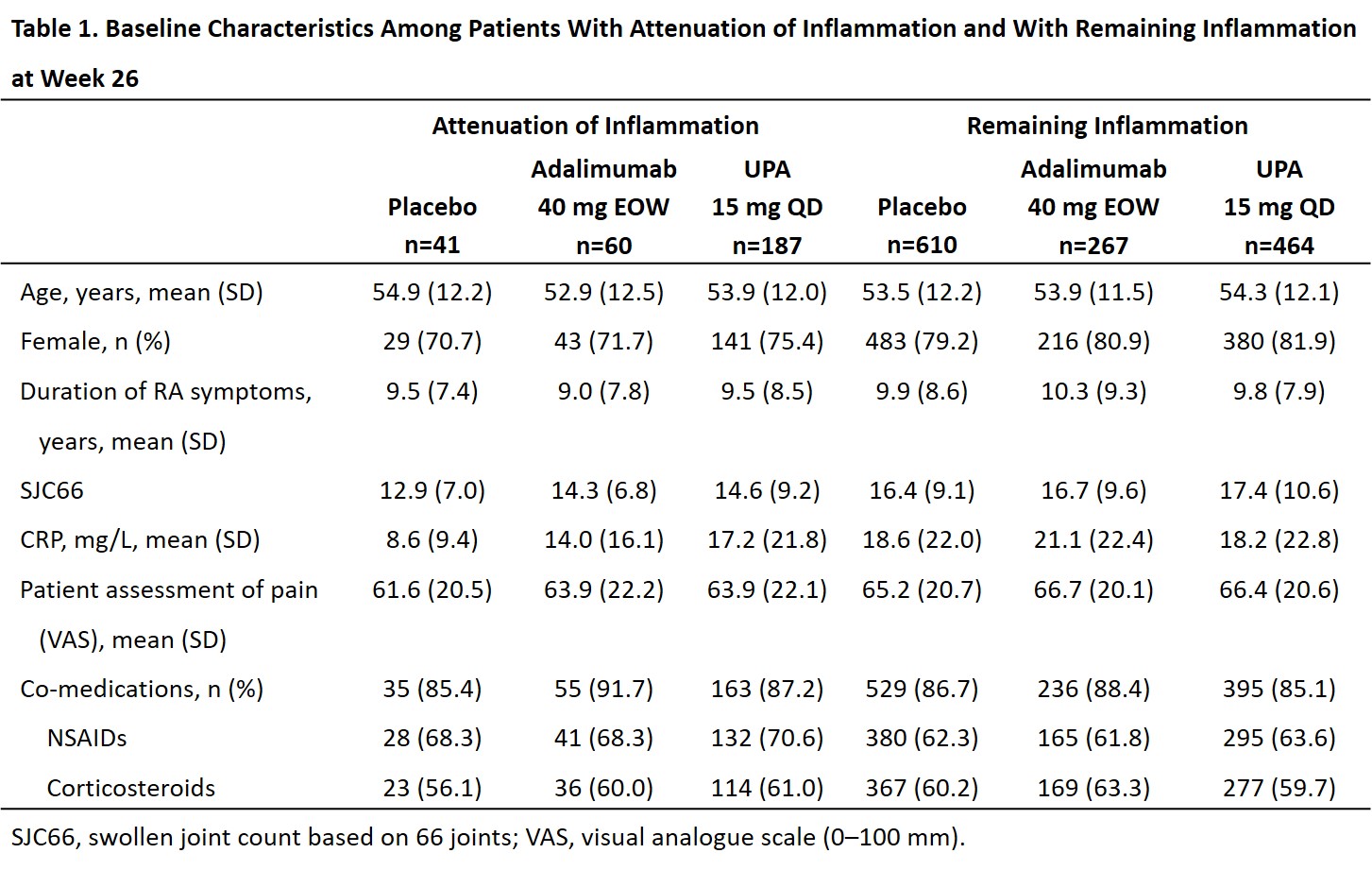
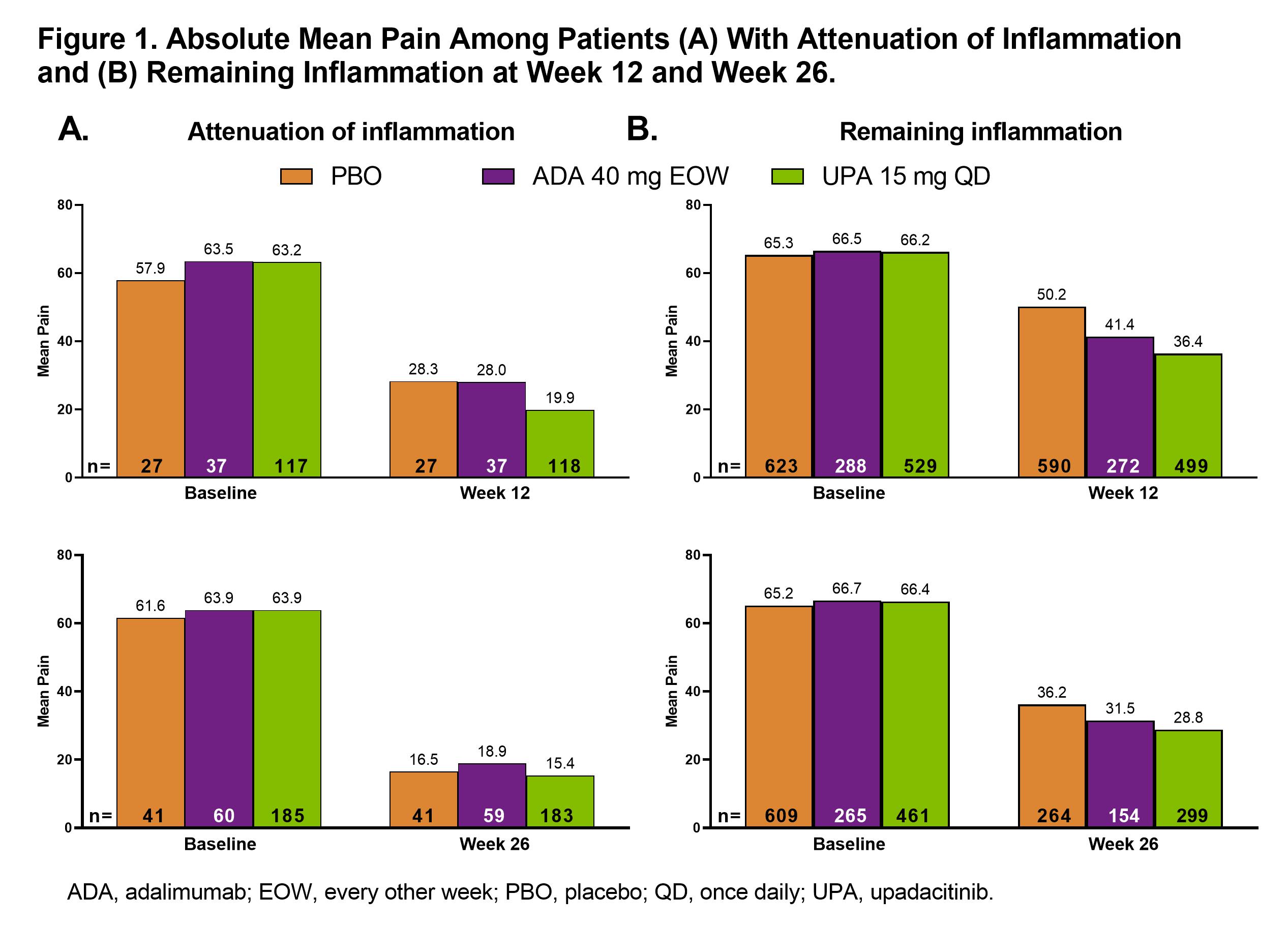
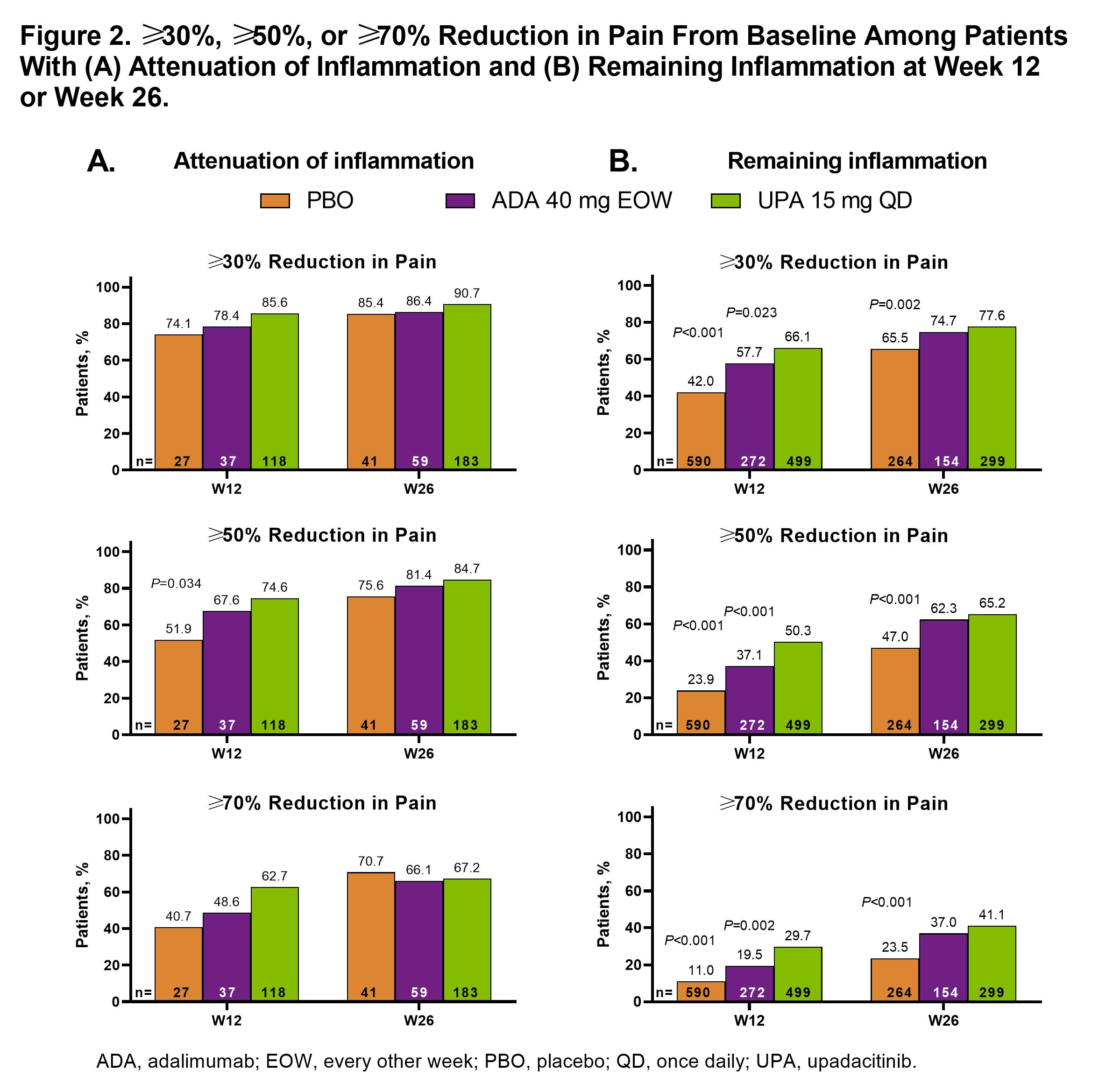
Results: Attenuation of inflammation at week 26 was reached by 41 (6.3%), 60 (18.3%), and 187 (28.7%) pts receiving PBO, ADA, and UPA, respectively; remaining inflammation was present in 610 (93.7%), 267 (81.7%), and 464 (71.3%) pts, respectively. Differences in baseline characteristics were observed (Table 1). Among pts with attenuation of inflammation, pain improved more with UPA (mean [95% CI]: –42.9 [–46.6, –39.1]) than ADA (–34.7 [–41.1, –28.0]; nominal P=0.038) or PBO (–33.1 [–41.0, –25.2]; nominal P=0.029) at week 12; change from baseline results were numerically similar at week 26. Absolute mean values for pain showed a similar trend (Figure 1A). Among pts with remaining inflammation, a similar trend was observed, which was significant for UPA vs PBO at weeks 12 and 26 and for UPA vs ADA at week 12 (Figure 1B). For ≥30%, ≥50%, or ≥70% reduction in pain from baseline, UPA had a numerically higher response rate than ADA or PBO at weeks 12 and 26 (significant for UPA vs PBO at week 12 for ≥50% reduction) in pts with attenuation of inflammation (Figure 2A). Among pts with remaining inflammation, significantly greater response rates were observed with UPA vs PBO at weeks 12 and 26 and vs ADA at week 12 for ≥30%, ≥50%, and ≥70% reductions in pain from baseline (Figure 2B).
Conclusion: Among both pts with attenuation of or remaining inflammation, pain improved more with UPA vs ADA or PBO at weeks 12 and 26. These results suggest that UPA is effective in reducing pain in pts with active RA who had attenuation of inflammation at 3 or 6 months.
Reference:
Disclosures: L. Bessette, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi-Genzyme, UCB, Gilead, Merck/MSD, Organon, Roche; A. Kakehasi, AbbVie, Amgen, Janssen, UCB, Pfizer, Eli Lilly, Novartis, Sandoz, Fresenius Kabi, Organon; N. Basu, Vifor, Pfizer, GSK, Nesos, Lilly, Roche, AbbVie, MSD, Galapagos; D. Walsh, GlaxoSmithKlein(GSK), AKL Research & Development Ltd, Pfizer Ltd, Eli Lilly and Company, Galapagos, AbbVie Ltd, UCB Pharma; A. Balanescu, AbbVie; C. Bingham, AbbVie, Janssen, Pfizer, Sanofi, BMS; A. Garrison, AbbVie Inc.; I. Lagunes, AbbVie Inc.; s. strengholt, AbbVie; R. Lippe, AbbVie; M. Dougados, AbbVie, Bristol-Myers Squibb(BMS), Eli Lilly, Merck, Novartis, Pfizer Inc., Roche, UCB.
Background/Purpose: Residual pain remains a challenge that prevents patients (pts) with RA from achieving a better quality of life. Current RA treatments have demonstrated efficacy in reducing pain. Janus kinase inhibitor upadacitinib (UPA)+background MTX demonstrated higher improvements in pain vs adalimumab (ADA)+MTX and placebo (PBO)+MTX in the SELECT-COMPARE study.1 The objective of this post-hoc analysis was to describe levels of pain reduction and residual pain after treatment with UPA+MTX vs ADA+MTX and PBO+MTX in pts with RA with and without attenuation of inflammation at weeks 12 and 26.
Methods: Pts with active RA receiving background MTX were randomized to UPA 15 mg once daily, ADA 40 mg every other week, or PBO. Subgroup assessment between pts with attenuation of inflammation (defined as swollen joint count based on 66 joints (SJC66) of 0 and CRP < 6 mg/L) vs remaining inflammation at week 12 and week 26 was conducted. Pain was measured by a visual analogue scale (0 ["no pain"] to 100 mm ["most severe pain"]). Mean change from baseline in pain at week 12 and week 26 as well as ≥30%, ≥50%, or ≥70% reduction from baseline to week 12 and week 26 in pain were assessed. Observed case data are reported in full analysis set, including all randomized pts who had received at least one dose of study drug. For change from baseline analyses, between group least squares mean, 95% confidence interval, and nominal P value were based on analysis of covariance model with treatment as a fixed factor and baseline value as a covariate. For binary endpoint analyses, nominal P values were calculated by the chi-square test.
Results: Attenuation of inflammation at week 26 was reached by 41 (6.3%), 60 (18.3%), and 187 (28.7%) pts receiving PBO, ADA, and UPA, respectively; remaining inflammation was present in 610 (93.7%), 267 (81.7%), and 464 (71.3%) pts, respectively. Differences in baseline characteristics were observed (Table 1). Among pts with attenuation of inflammation, pain improved more with UPA (mean [95% CI]: –42.9 [–46.6, –39.1]) than ADA (–34.7 [–41.1, –28.0]; nominal P=0.038) or PBO (–33.1 [–41.0, –25.2]; nominal P=0.029) at week 12; change from baseline results were numerically similar at week 26. Absolute mean values for pain showed a similar trend (Figure 1A). Among pts with remaining inflammation, a similar trend was observed, which was significant for UPA vs PBO at weeks 12 and 26 and for UPA vs ADA at week 12 (Figure 1B). For ≥30%, ≥50%, or ≥70% reduction in pain from baseline, UPA had a numerically higher response rate than ADA or PBO at weeks 12 and 26 (significant for UPA vs PBO at week 12 for ≥50% reduction) in pts with attenuation of inflammation (Figure 2A). Among pts with remaining inflammation, significantly greater response rates were observed with UPA vs PBO at weeks 12 and 26 and vs ADA at week 12 for ≥30%, ≥50%, and ≥70% reductions in pain from baseline (Figure 2B).
Conclusion: Among both pts with attenuation of or remaining inflammation, pain improved more with UPA vs ADA or PBO at weeks 12 and 26. These results suggest that UPA is effective in reducing pain in pts with active RA who had attenuation of inflammation at 3 or 6 months.
Reference:
1. Fleischmann RM, et al. Arthritis Rheumatol. 2019;71(11):1788-1800.



Disclosures: L. Bessette, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi-Genzyme, UCB, Gilead, Merck/MSD, Organon, Roche; A. Kakehasi, AbbVie, Amgen, Janssen, UCB, Pfizer, Eli Lilly, Novartis, Sandoz, Fresenius Kabi, Organon; N. Basu, Vifor, Pfizer, GSK, Nesos, Lilly, Roche, AbbVie, MSD, Galapagos; D. Walsh, GlaxoSmithKlein(GSK), AKL Research & Development Ltd, Pfizer Ltd, Eli Lilly and Company, Galapagos, AbbVie Ltd, UCB Pharma; A. Balanescu, AbbVie; C. Bingham, AbbVie, Janssen, Pfizer, Sanofi, BMS; A. Garrison, AbbVie Inc.; I. Lagunes, AbbVie Inc.; s. strengholt, AbbVie; R. Lippe, AbbVie; M. Dougados, AbbVie, Bristol-Myers Squibb(BMS), Eli Lilly, Merck, Novartis, Pfizer Inc., Roche, UCB.

