Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0343–0371) SLE – Treatment Poster I
0364: Efficacy of BIIB059 on Joint and Skin Manifestations in Participants with Systemic Lupus Erythematosus (SLE): Exploratory Analyses of the Phase 2, Randomized, Double-Blind, Placebo-Controlled LILAC Study (Part A)
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall

Richard Furie, MD
Chief of the Division of Rheumatology
Northwell Health
Manhasset, NY, NY, United States
Abstract Poster Presenter(s)
Richard Furie1, Ronald van Vollenhoven2, Victoria Werth3, Kenneth Kalunian4, Sandra Navarra5, Juanita Romero-Diaz6, Ting Wang7, Cristina Musselli7, Catherine Barbey8 and Nathalie Franchimont7, 1Northwell Health, Great Neck, NY, 2Amsterdam University Medical Centers, Amsterdam, Netherlands, 3University of Pennsylvania and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, PA, 4University of California San Diego, La Jolla, CA, 5University of Santo Tomas, Manila, Philippines, 6Instituto Nacional de Ciencias Medicas y Nutricion SZ, Ciudad de México, Mexico, 7Biogen, Cambridge, MA, 8Biogen, Baar, Switzerland
Background/Purpose: SLE is a heterogeneous illness that often manifests with skin and/or joint diseases.1 BIIB059 is a humanized monoclonal antibody that binds to BDCA2, a receptor exclusively expressed on plasmacytoid dendritic cells, inhibiting the production of Type I interferons, cytokines and chemokines.2 Part A of the LILAC study (NCT02847598) met its primary endpoint, with a significantly greater reduction in total active joint count with BIIB059 vs placebo (PBO) at Week 24.3 Additional exploratory analyses evaluating changes in joint and skin disease activity are presented here.
Methods: Adults with SLE and active skin and joint manifestations were randomized to receive BIIB059 450 mg (n=64) or PBO (n=56) subcutaneously at Weeks 2, 4, 8, 12, 16, and 20. Improvements in joint and skin disease activity were assessed using several endpoints. The proportions of participants (pts) attaining a ≥50% reduction from baseline (BL) in active joint count using a modified definition (Joint-50 response, post-hoc analysis), or in Cutaneous Lupus Erythematosus Disease Area and Severity Index – Activity (CLASI-A) score (CLASI-50 response, prespecified exploratory analysis), or a ≥7-point reduction from BL in CLASI-A score (prespecified exploratory analysis) were assessed over time. Resolution of arthritis, rash, or both arthritis and rash by the SLE Disease Activity Index 2000 (SLEDAI-2K) was assessed at Week 24 (all ad-hoc analyses). Improvements from Grade A or B at BL in the British Isles Lupus Assessment Group Index 2004 (BILAG-2004) musculoskeletal, mucocutaneous, or both musculoskeletal and mucocutaneous domains were assessed at Week 24 (all ad-hoc analyses). Data are summarized descriptively.
Results: Greater proportions of BIIB059- vs PBO-treated pts achieved Joint-50 responses, CLASI‑50 responses, or a ≥7-point reduction from BL in CLASI-A score over time (Figure 1). At Week 24, greater proportions of BIIB059-treated pts had resolution of SLEDAI-2K arthritis or rash compared with PBO (Figure 2). A greater proportion of BIIB059- vs PBO‑treated pts had resolution of both SLEDAI-2K domains at Week 24 (14/61 [23.0%] vs 5/52 [9.6%]). Greater proportions of BIIB059-treated pts improved from Grade A or B at BL to Grade C or D at Week 24 in the BILAG-2004 mucocutaneous or musculoskeletal domains, compared with PBO (Figure 3). Of pts with Grade A or B at BL in both BILAG-2004 domains, a greater proportion of BIIB059- vs PBO-treated pts improved by at least 1 grade in both domains at Week 24 (27/54, 50.0% vs 15/44, 34.1%).
Conclusion: Among pts with SLE with joint and skin activity at study entry, those receiving BIIB059 had consistent improvements vs PBO in joint, skin, or both manifestations. These data suggest a potential benefit of BIIB059 treatment for joint and skin manifestations in patients with SLE. Two Phase 3 studies of BIIB059 are currently ongoing.
References:
1. Fava A, Petri M. J Autoimmun 2019;96:1–13
2. Pellerin A, et al. EMBO Mol Med 2015;7:464–476
3. Furie R, et al. Arthritis Rheumatol 2020;72(Suppl. 10):0935 (Abs.)
Funding: This study was funded by Biogen (Cambridge, MA, USA). Writing and editorial support were provided by Selene Medical Communications (Macclesfield, UK), funded by Biogen.
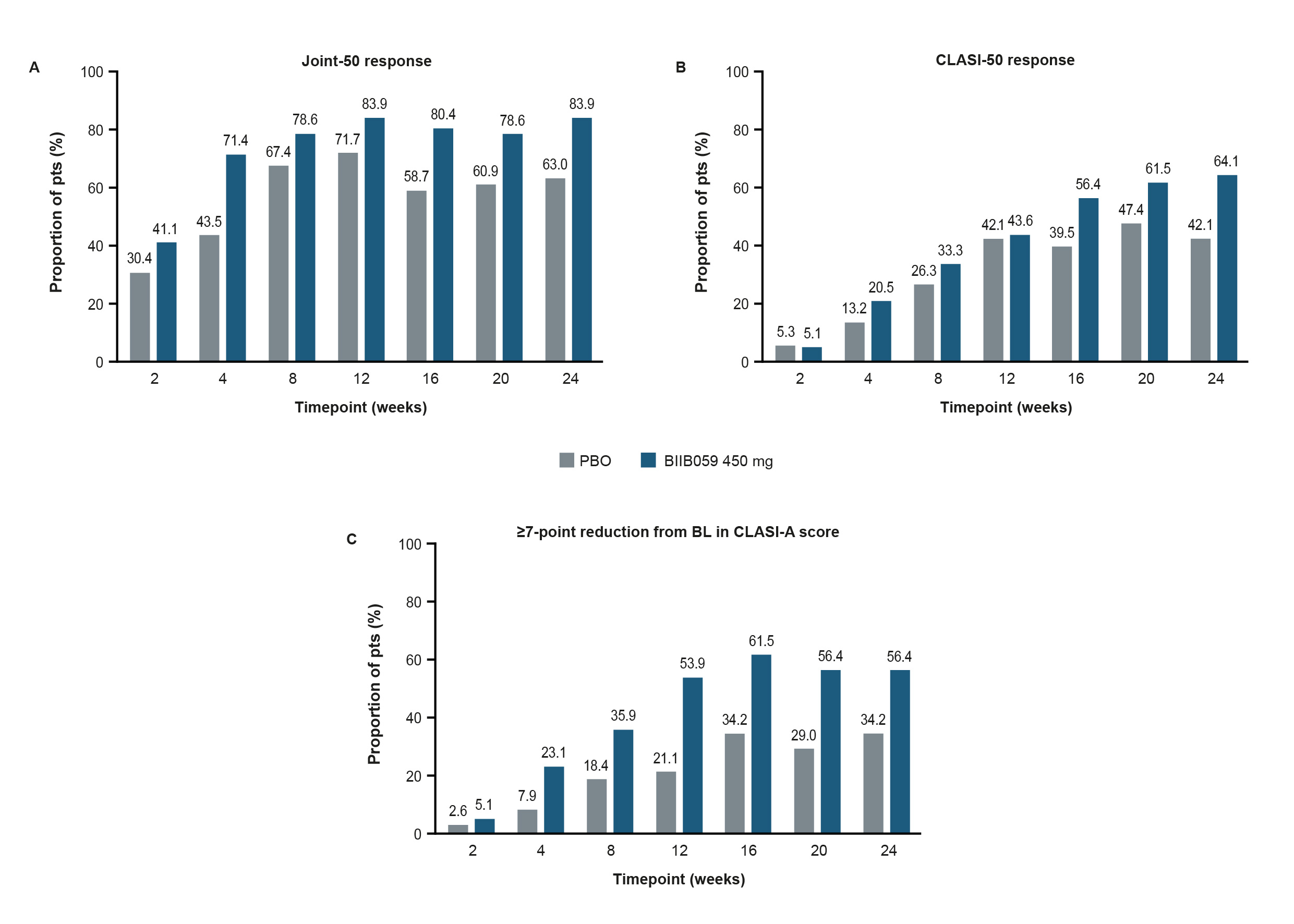 Figure 1. Proportions of pts with (A) a Joint-50 response, (B) a CLASI-50 response, or (C) a ≥7-point reduction from BL in CLASI-A score over time.
Figure 1. Proportions of pts with (A) a Joint-50 response, (B) a CLASI-50 response, or (C) a ≥7-point reduction from BL in CLASI-A score over time.
Figure 1A presents the proportion of pts with a ≥50% reduction from BL in active joint count using a modified definition, which was evaluated by the 28-joint assessment and defined as the sum of joints that were both tender and swollen. Pts included in this post-hoc analysis had ≥4 tender and ≥4 swollen joints at BL, with ≥4 of the swollen joints occurring in the proximal interphalangeal, metacarpophalangeal, or wrist joints. In Figure 1B and C, only pts with a BL CLASI-A score of ≥8 were included in these prespecified exploratory analyses. In Figure 1A–C, pts who experienced treatment failure or who discontinued treatment were considered to be non-responders.
BL, baseline; CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; CLASI-A, CLASI-Activity; PBO, placebo; pts, participants
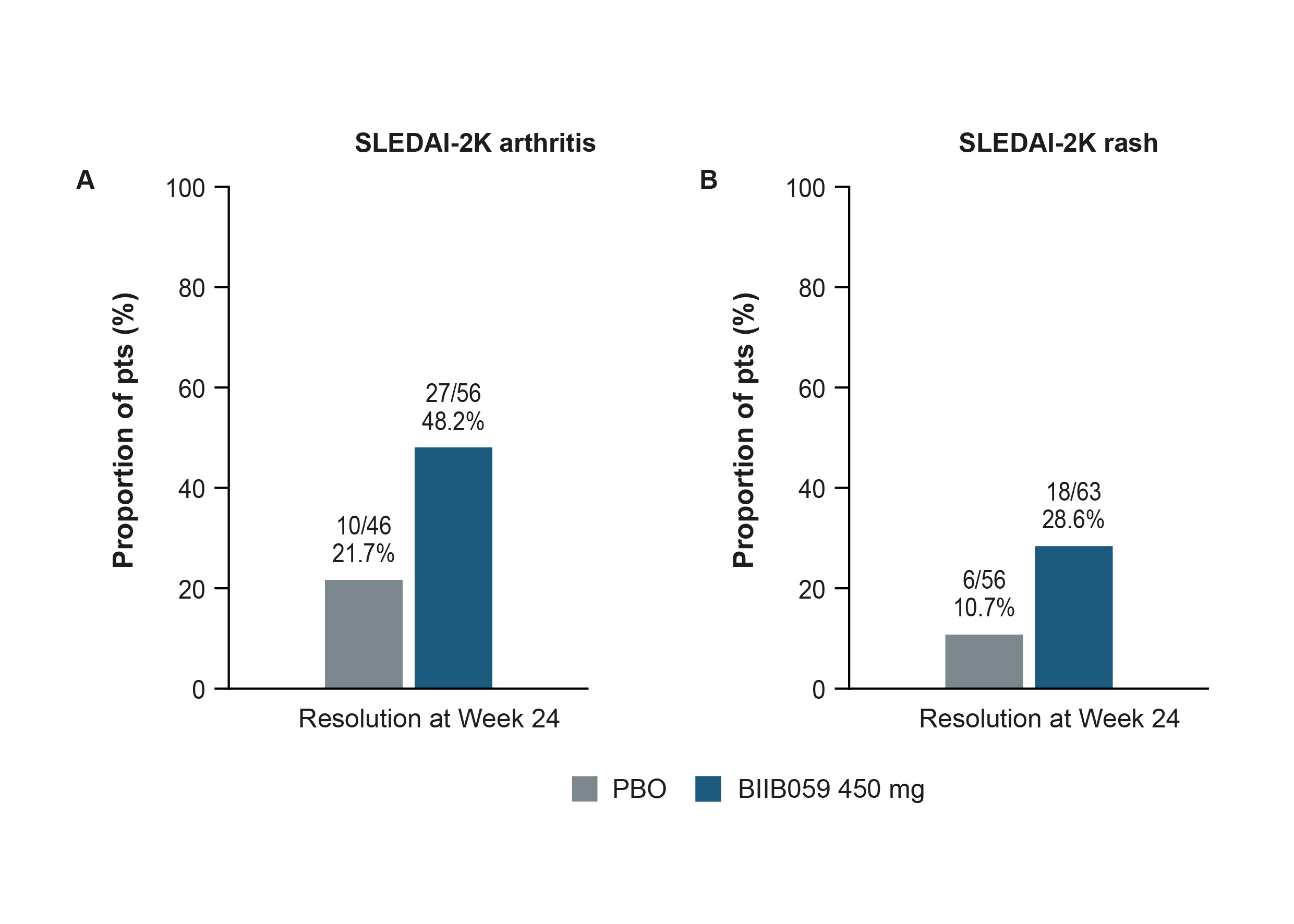 Figure 2. Proportions of pts with resolution of (A) arthritis or (B) rash by SLEDAI-2K at Week 24.
Figure 2. Proportions of pts with resolution of (A) arthritis or (B) rash by SLEDAI-2K at Week 24.
Resolution was defined as a change from ‘Present’ at BL to ‘Absent’ at Week 24 in SLEDAI‑2K arthritis (A) or rash (B). Only pts with a positive arthritis (A) or rash (B) item on the SLEDAI-2K questionnaire at BL were included in these analyses. Data from pts enrolled under protocol version 1 were only included in the arthritis analysis if, based on assessment of 28 joints, they had ≥4 tender and ≥4 swollen joints at BL, with ≥4 of the swollen joints occurring in the proximal interphalangeal, metacarpophalangeal, or wrist joints. Pts who experienced treatment failure or who discontinued treatment were considered as not having resolution; such pts were considered as non-responders at visits following treatment failure or discontinuation. Pts who completed treatment but had a missing score at any primary timepoint are classified as non-responders for that timepoint.
BL, baseline; PBO, placebo; pts, participants; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000
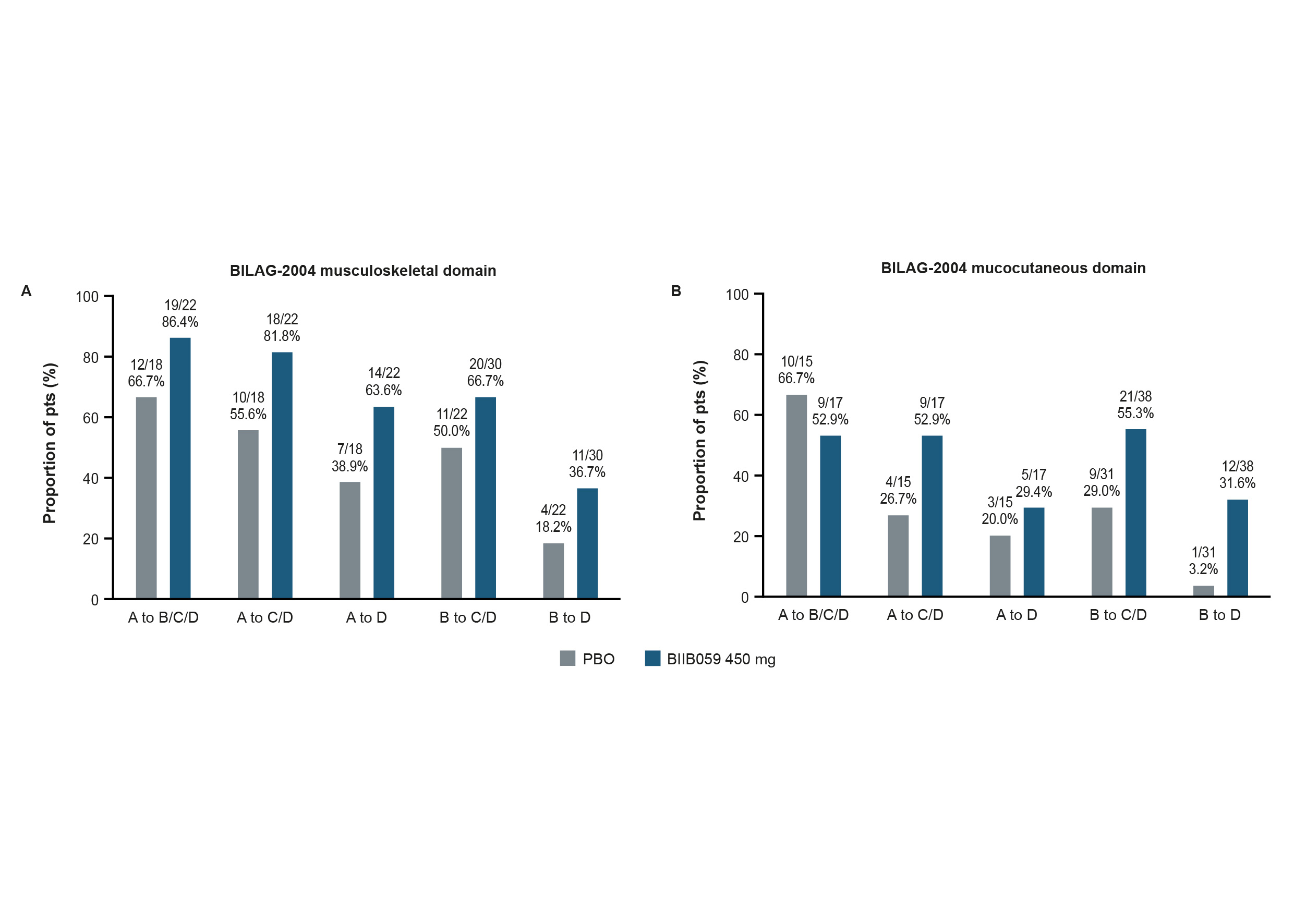 Figure 3. Proportions of pts with an improvement from BL in the BILAG-2004 (A) musculoskeletal or (B) mucocutaneous domains at Week 24.
Figure 3. Proportions of pts with an improvement from BL in the BILAG-2004 (A) musculoskeletal or (B) mucocutaneous domains at Week 24.
Only pts with Grade A or B at BL in the BILAG-2004 musculoskeletal (A) or mucocutaneous (B) domains were included in these ad-hoc analyses. Missing data were imputed via last observation carried forward, with the worse of BL or last visit before treatment failure carried forward for pts who experienced treatment failure. Pts were censored after treatment discontinuation.
BILAG-2004, British Isles Lupus Assessment Group Index 2004; BL, baseline; PBO, placebo; pts, participants
Disclosures: R. Furie, AstraZeneca, Biogen; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; V. Werth, AbbVie/Abbott, Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Celgene, Eli Lilly, Genentech, GlaxoSmithKline (GSK), Janssen, Merck/MSD, Gilead, Novartis, Pfizer, Rome Pharmaceuticals, Horizon Therapeutics, Regeneron, argenx, CSL Behring, AnaptysBio, Biogen, Corbus, EMD Serono, Galderma, Nektar, Octapharma, Principia, Resolve, Sanofi, Syntimmune, Viela; K. Kalunian, AbbVie/Abbott, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb (BMS), Eli Lilly, Equillium, Genentech, Gilead, Janssen, Roche, Lupus Research Alliance, Pfizer, Sanford Consortium, Viela, Nektar; S. Navarra, Biogen, Astellas, Janssen, Novartis, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline (GSK); J. Romero-Diaz, Biogen; T. Wang, Biogen; C. Musselli, Biogen; C. Barbey, Biogen; N. Franchimont, Biogen, OMass Therapeutics, Alexion (spouse), AstraZeneca (spouse).
Background/Purpose: SLE is a heterogeneous illness that often manifests with skin and/or joint diseases.1 BIIB059 is a humanized monoclonal antibody that binds to BDCA2, a receptor exclusively expressed on plasmacytoid dendritic cells, inhibiting the production of Type I interferons, cytokines and chemokines.2 Part A of the LILAC study (NCT02847598) met its primary endpoint, with a significantly greater reduction in total active joint count with BIIB059 vs placebo (PBO) at Week 24.3 Additional exploratory analyses evaluating changes in joint and skin disease activity are presented here.
Methods: Adults with SLE and active skin and joint manifestations were randomized to receive BIIB059 450 mg (n=64) or PBO (n=56) subcutaneously at Weeks 2, 4, 8, 12, 16, and 20. Improvements in joint and skin disease activity were assessed using several endpoints. The proportions of participants (pts) attaining a ≥50% reduction from baseline (BL) in active joint count using a modified definition (Joint-50 response, post-hoc analysis), or in Cutaneous Lupus Erythematosus Disease Area and Severity Index – Activity (CLASI-A) score (CLASI-50 response, prespecified exploratory analysis), or a ≥7-point reduction from BL in CLASI-A score (prespecified exploratory analysis) were assessed over time. Resolution of arthritis, rash, or both arthritis and rash by the SLE Disease Activity Index 2000 (SLEDAI-2K) was assessed at Week 24 (all ad-hoc analyses). Improvements from Grade A or B at BL in the British Isles Lupus Assessment Group Index 2004 (BILAG-2004) musculoskeletal, mucocutaneous, or both musculoskeletal and mucocutaneous domains were assessed at Week 24 (all ad-hoc analyses). Data are summarized descriptively.
Results: Greater proportions of BIIB059- vs PBO-treated pts achieved Joint-50 responses, CLASI‑50 responses, or a ≥7-point reduction from BL in CLASI-A score over time (Figure 1). At Week 24, greater proportions of BIIB059-treated pts had resolution of SLEDAI-2K arthritis or rash compared with PBO (Figure 2). A greater proportion of BIIB059- vs PBO‑treated pts had resolution of both SLEDAI-2K domains at Week 24 (14/61 [23.0%] vs 5/52 [9.6%]). Greater proportions of BIIB059-treated pts improved from Grade A or B at BL to Grade C or D at Week 24 in the BILAG-2004 mucocutaneous or musculoskeletal domains, compared with PBO (Figure 3). Of pts with Grade A or B at BL in both BILAG-2004 domains, a greater proportion of BIIB059- vs PBO-treated pts improved by at least 1 grade in both domains at Week 24 (27/54, 50.0% vs 15/44, 34.1%).
Conclusion: Among pts with SLE with joint and skin activity at study entry, those receiving BIIB059 had consistent improvements vs PBO in joint, skin, or both manifestations. These data suggest a potential benefit of BIIB059 treatment for joint and skin manifestations in patients with SLE. Two Phase 3 studies of BIIB059 are currently ongoing.
References:
1. Fava A, Petri M. J Autoimmun 2019;96:1–13
2. Pellerin A, et al. EMBO Mol Med 2015;7:464–476
3. Furie R, et al. Arthritis Rheumatol 2020;72(Suppl. 10):0935 (Abs.)
Funding: This study was funded by Biogen (Cambridge, MA, USA). Writing and editorial support were provided by Selene Medical Communications (Macclesfield, UK), funded by Biogen.
 Figure 1. Proportions of pts with (A) a Joint-50 response, (B) a CLASI-50 response, or (C) a ≥7-point reduction from BL in CLASI-A score over time.
Figure 1. Proportions of pts with (A) a Joint-50 response, (B) a CLASI-50 response, or (C) a ≥7-point reduction from BL in CLASI-A score over time. Figure 1A presents the proportion of pts with a ≥50% reduction from BL in active joint count using a modified definition, which was evaluated by the 28-joint assessment and defined as the sum of joints that were both tender and swollen. Pts included in this post-hoc analysis had ≥4 tender and ≥4 swollen joints at BL, with ≥4 of the swollen joints occurring in the proximal interphalangeal, metacarpophalangeal, or wrist joints. In Figure 1B and C, only pts with a BL CLASI-A score of ≥8 were included in these prespecified exploratory analyses. In Figure 1A–C, pts who experienced treatment failure or who discontinued treatment were considered to be non-responders.
BL, baseline; CLASI, Cutaneous Lupus Erythematosus Disease Area and Severity Index; CLASI-A, CLASI-Activity; PBO, placebo; pts, participants
 Figure 2. Proportions of pts with resolution of (A) arthritis or (B) rash by SLEDAI-2K at Week 24.
Figure 2. Proportions of pts with resolution of (A) arthritis or (B) rash by SLEDAI-2K at Week 24.Resolution was defined as a change from ‘Present’ at BL to ‘Absent’ at Week 24 in SLEDAI‑2K arthritis (A) or rash (B). Only pts with a positive arthritis (A) or rash (B) item on the SLEDAI-2K questionnaire at BL were included in these analyses. Data from pts enrolled under protocol version 1 were only included in the arthritis analysis if, based on assessment of 28 joints, they had ≥4 tender and ≥4 swollen joints at BL, with ≥4 of the swollen joints occurring in the proximal interphalangeal, metacarpophalangeal, or wrist joints. Pts who experienced treatment failure or who discontinued treatment were considered as not having resolution; such pts were considered as non-responders at visits following treatment failure or discontinuation. Pts who completed treatment but had a missing score at any primary timepoint are classified as non-responders for that timepoint.
BL, baseline; PBO, placebo; pts, participants; SLEDAI-2K, Systemic Lupus Erythematosus Disease Activity Index 2000
 Figure 3. Proportions of pts with an improvement from BL in the BILAG-2004 (A) musculoskeletal or (B) mucocutaneous domains at Week 24.
Figure 3. Proportions of pts with an improvement from BL in the BILAG-2004 (A) musculoskeletal or (B) mucocutaneous domains at Week 24.Only pts with Grade A or B at BL in the BILAG-2004 musculoskeletal (A) or mucocutaneous (B) domains were included in these ad-hoc analyses. Missing data were imputed via last observation carried forward, with the worse of BL or last visit before treatment failure carried forward for pts who experienced treatment failure. Pts were censored after treatment discontinuation.
BILAG-2004, British Isles Lupus Assessment Group Index 2004; BL, baseline; PBO, placebo; pts, participants
Disclosures: R. Furie, AstraZeneca, Biogen; R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; V. Werth, AbbVie/Abbott, Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Celgene, Eli Lilly, Genentech, GlaxoSmithKline (GSK), Janssen, Merck/MSD, Gilead, Novartis, Pfizer, Rome Pharmaceuticals, Horizon Therapeutics, Regeneron, argenx, CSL Behring, AnaptysBio, Biogen, Corbus, EMD Serono, Galderma, Nektar, Octapharma, Principia, Resolve, Sanofi, Syntimmune, Viela; K. Kalunian, AbbVie/Abbott, Amgen, AstraZeneca, Aurinia, Biogen, Bristol Myers Squibb (BMS), Eli Lilly, Equillium, Genentech, Gilead, Janssen, Roche, Lupus Research Alliance, Pfizer, Sanford Consortium, Viela, Nektar; S. Navarra, Biogen, Astellas, Janssen, Novartis, Pfizer, Boehringer-Ingelheim, GlaxoSmithKline (GSK); J. Romero-Diaz, Biogen; T. Wang, Biogen; C. Musselli, Biogen; C. Barbey, Biogen; N. Franchimont, Biogen, OMass Therapeutics, Alexion (spouse), AstraZeneca (spouse).

