Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0419: Efficacy of Upadacitinib in Patients with Non-Radiographic Axial Spondyloarthritis Stratified by Objective Signs of Inflammation at Baseline
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- WM
Walter P. Maksymowych, MD
University of Alberta
Edmonton, AB, Canada
Abstract Poster Presenter(s)
Walter P Maksymowych1, Xenofon Baraliakos2, Atul Deodhar3, Denis Poddubnyy4, Fabiana Ganz5, Tianming Gao6, Jayne Stigler6, Anna K Shmagel6, Peter Wung6 and Filip Van den bosch7, 1Department of Medicine, University of Alberta, Edmonton, AB, Canada, 2Rheumazentrum Ruhrgebiet Herne, Herne, Germany, 3Oregon Health & Science University, Portland, OR, USA, Portland, OR, 4Department of Gastroenterology, Infectious Diseases and Rheumatology, Charité – Universitätsmedizin Berlin, Berlin, Germany, 5AbbVie, Inc., Luzern, Switzerland, 6AbbVie, Inc., North Chicago, IL, 7Department of Internal Medicine and Paediatrics, Ghent University and VIB Centre for Inflammation Research, Ghent, Belgium
Background/Purpose: The Phase 3 SELECT-AXIS 2 trial (NCT04169373) assessed the efficacy and safety of upadacitinib (UPA) in patients with non-radiographic axial spondyloarthritis (nr-axSpA). Here, we present a subgroup analysis of the SELECT-AXIS 2 efficacy data stratified by elevated high-sensitivity CRP (hsCRP) and positivity for MRI inflammation in the sacroiliac (SI) joints at screening.
Methods: In SELECT-AXIS 2,1 patients aged ≥18 years with a clinical diagnosis of nr-axSpA, meeting the 2009 Assessment of Spondyloarthritis international Society (ASAS) classification criteria for axSpA, but not the radiologic criterion of the modified New York criteria for AS, and with objective signs of active inflammation on MRI per the ASAS definition (assessed by two primary readers and an adjudicator) and/or hsCRP exceeding the upper limit of normal (ULN, 2.87 mg/L) at screening, were randomized 1:1 to UPA 15 mg once daily or placebo (PBO). The primary endpoint, measured at Week (Wk) 14, was the ASAS40 response. Additional endpoints included Ankylosing Spondylitis Disease Activity Score (ASDAS) Low Disease Activity (LDA) with a score ≤2.1, change from baseline (BL) in MRI Spondyloarthritis Research Consortium of Canada (SPARCC) score (SI joints), BASFI, and patients' assessments of total back pain, all at Wk 14. Prespecified (ASAS40 response) and post hoc (all other endpoints) subgroup analyses were carried out by inflammatory status, with hsCRP ( >ULN vs ≤ULN) and MRI SI joint inflammation status (positive vs negative) at screening as stratification factors. A separate subgroup analysis by BL hsCRP status alone is also presented. Non-response imputation (NRI) with multiple imputation (MI), incorporating MI to handle missing data due to COVID-19, was used for binary outcomes. Mixed model for repeated measures based on as-observed (AO) data was used for continuous outcomes except MRI SPARCC score. Analysis of covariance based on AO data was used for MRI SPARCC score.
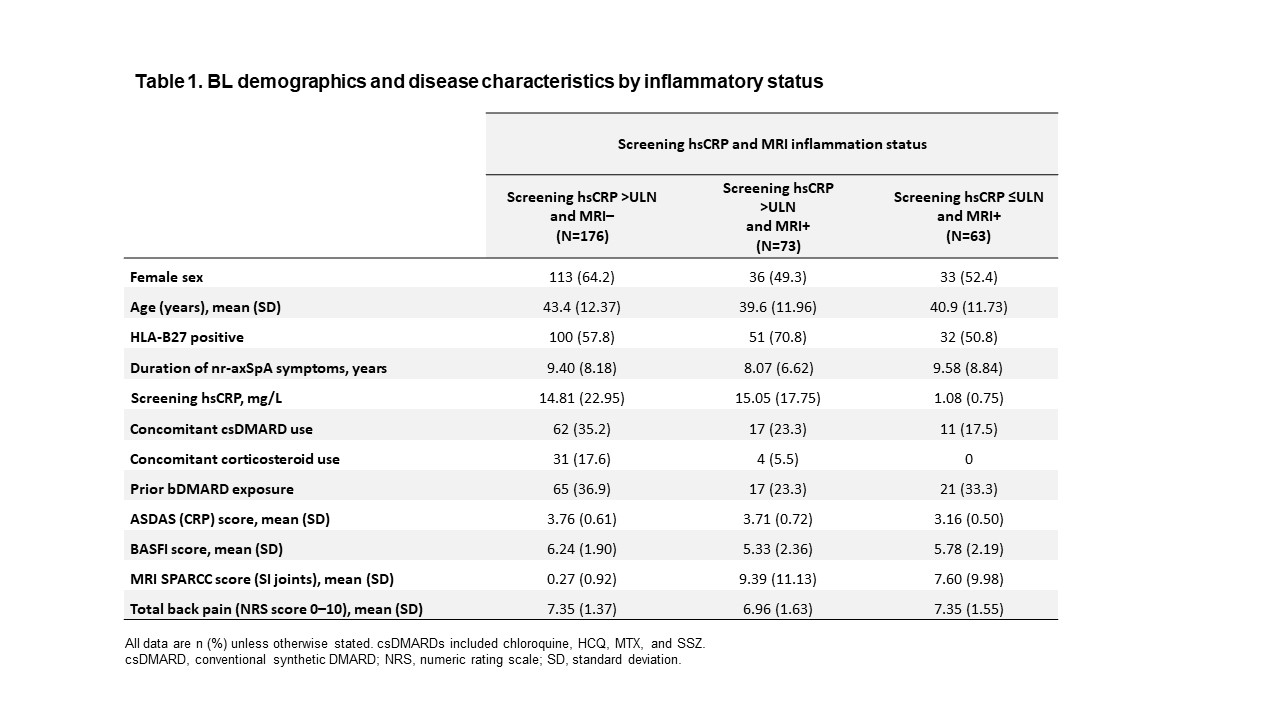
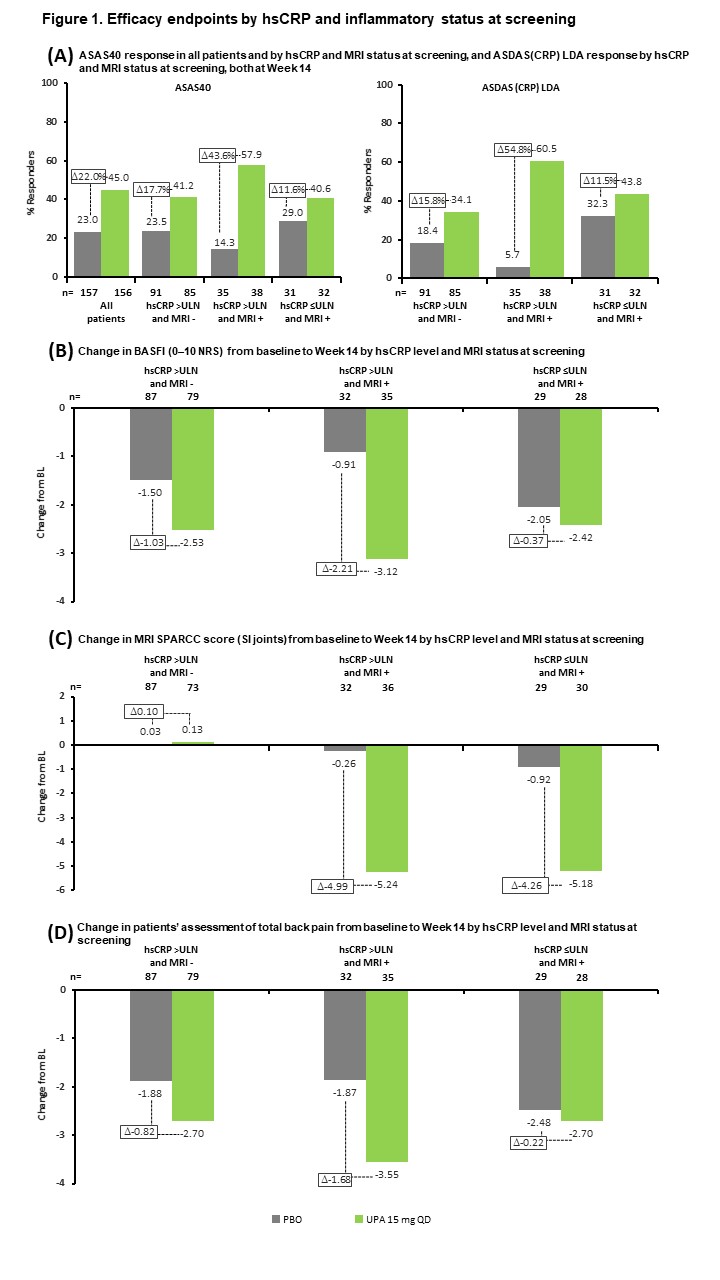
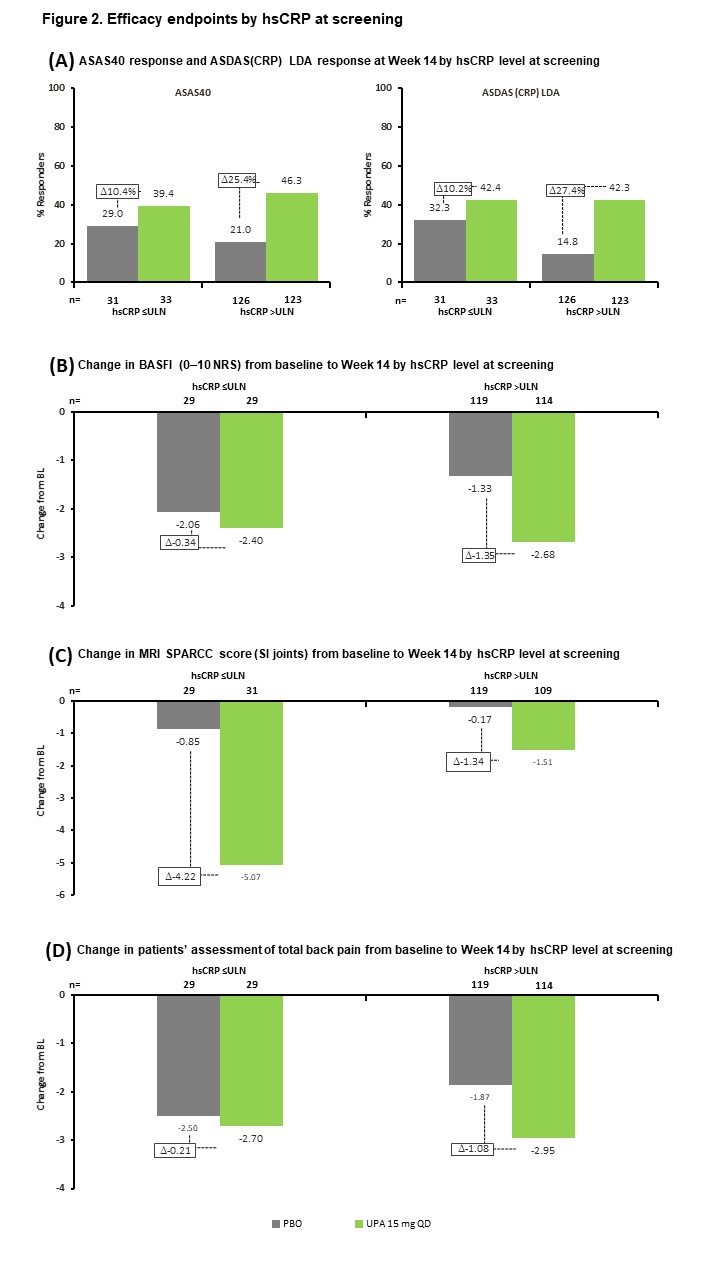
Results: Of 312 patients included in the analysis, 176 (56%) had hsCRP >ULN and were MRI negative (MRI−), 73 (23%) had hsCRP >ULN and were MRI positive (MRI+), and 63 (20%) had hsCRP ≤ULN and were MRI+. BL demographics and disease characteristics were generally similar across subgroups, although the hsCRP >ULN and MRI+ subgroup had a higher frequency of HLA-B27-positive patients and a lower rate of prior biologic DMARD (bDMARD) exposure than the other subgroups (Table 1). UPA was associated with increased ASAS40 and ASDAS LDA rates, and greater reductions in MRI SPARCC score, BASFI, and total back pain from BL, vs PBO across all subgroups at Wk 14 (Figure 1). The treatment difference between UPA and PBO was greatest in the hsCRP >ULN and MRI+ group for all endpoints. When considering BL hsCRP alone, the treatment difference was greater in the hsCRP >ULN group vs the hsCRP ≤ULN subgroup for all endpoints except change from BL in MRI SPARCC score (SI joints) (Figure 2).
Conclusion: In SELECT-AXIS 2, UPA improved outcomes vs PBO in patients with nr-axSpA across all BL inflammation subgroups; the greatest benefit was observed in patients with both elevated CRP levels and evidence of inflammation on MRI at screening.
Reference 1. Deodhar A, et al. Ann Rheum Dis 2022;81(Suppl 1):9. Abstract OP0016
Disclosures: W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; A. Deodhar, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer Inc, UCB Pharma, Aurinia, Moonlake; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB; F. Ganz, AbbVie; T. Gao, AbbVie; J. Stigler, AbbVie; A. Shmagel, AbbVie; P. Wung, AbbVie; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene.
Background/Purpose: The Phase 3 SELECT-AXIS 2 trial (NCT04169373) assessed the efficacy and safety of upadacitinib (UPA) in patients with non-radiographic axial spondyloarthritis (nr-axSpA). Here, we present a subgroup analysis of the SELECT-AXIS 2 efficacy data stratified by elevated high-sensitivity CRP (hsCRP) and positivity for MRI inflammation in the sacroiliac (SI) joints at screening.
Methods: In SELECT-AXIS 2,1 patients aged ≥18 years with a clinical diagnosis of nr-axSpA, meeting the 2009 Assessment of Spondyloarthritis international Society (ASAS) classification criteria for axSpA, but not the radiologic criterion of the modified New York criteria for AS, and with objective signs of active inflammation on MRI per the ASAS definition (assessed by two primary readers and an adjudicator) and/or hsCRP exceeding the upper limit of normal (ULN, 2.87 mg/L) at screening, were randomized 1:1 to UPA 15 mg once daily or placebo (PBO). The primary endpoint, measured at Week (Wk) 14, was the ASAS40 response. Additional endpoints included Ankylosing Spondylitis Disease Activity Score (ASDAS) Low Disease Activity (LDA) with a score ≤2.1, change from baseline (BL) in MRI Spondyloarthritis Research Consortium of Canada (SPARCC) score (SI joints), BASFI, and patients' assessments of total back pain, all at Wk 14. Prespecified (ASAS40 response) and post hoc (all other endpoints) subgroup analyses were carried out by inflammatory status, with hsCRP ( >ULN vs ≤ULN) and MRI SI joint inflammation status (positive vs negative) at screening as stratification factors. A separate subgroup analysis by BL hsCRP status alone is also presented. Non-response imputation (NRI) with multiple imputation (MI), incorporating MI to handle missing data due to COVID-19, was used for binary outcomes. Mixed model for repeated measures based on as-observed (AO) data was used for continuous outcomes except MRI SPARCC score. Analysis of covariance based on AO data was used for MRI SPARCC score.
Results: Of 312 patients included in the analysis, 176 (56%) had hsCRP >ULN and were MRI negative (MRI−), 73 (23%) had hsCRP >ULN and were MRI positive (MRI+), and 63 (20%) had hsCRP ≤ULN and were MRI+. BL demographics and disease characteristics were generally similar across subgroups, although the hsCRP >ULN and MRI+ subgroup had a higher frequency of HLA-B27-positive patients and a lower rate of prior biologic DMARD (bDMARD) exposure than the other subgroups (Table 1). UPA was associated with increased ASAS40 and ASDAS LDA rates, and greater reductions in MRI SPARCC score, BASFI, and total back pain from BL, vs PBO across all subgroups at Wk 14 (Figure 1). The treatment difference between UPA and PBO was greatest in the hsCRP >ULN and MRI+ group for all endpoints. When considering BL hsCRP alone, the treatment difference was greater in the hsCRP >ULN group vs the hsCRP ≤ULN subgroup for all endpoints except change from BL in MRI SPARCC score (SI joints) (Figure 2).
Conclusion: In SELECT-AXIS 2, UPA improved outcomes vs PBO in patients with nr-axSpA across all BL inflammation subgroups; the greatest benefit was observed in patients with both elevated CRP levels and evidence of inflammation on MRI at screening.
Reference 1. Deodhar A, et al. Ann Rheum Dis 2022;81(Suppl 1):9. Abstract OP0016



Disclosures: W. Maksymowych, AbbVie, Boehringer-Ingelheim, Celgene, Eli Lilly, Galapagos, Janssen, Novartis, Pfizer, UCB, CARE Arthritis Limited; X. Baraliakos, AbbVie, Lilly, Galapagos, MSD, Novartis, Pfizer, UCB, Bristol-Myers Squibb, Janssen, Roche, Sandoz, Sanofi; A. Deodhar, AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, GlaxoSmithKline, Janssen, Novartis, Pfizer Inc, UCB Pharma, Aurinia, Moonlake; D. Poddubnyy, AbbVie, Biocad, Bristol-Myers Squibb, Eli Lilly, Gilead, GlaxoSmithKline, MSD, Moonlake, Novartis, Pfizer, Samsung-Bioepis, UCB; F. Ganz, AbbVie; T. Gao, AbbVie; J. Stigler, AbbVie; A. Shmagel, AbbVie; P. Wung, AbbVie; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene.

