Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0241–0271) RA – Diagnosis, Manifestations, and Outcomes Poster I
0255: Is Abatacept as Effective and Safe in Rheumatoid Arthritis Patients with Previous Malignancy as in Those Without?
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- YK
Yosuke Kunishita, MD, PhD
Department of Rheumatology, Yokohama Minami Kyosai Hospital
Yokohama, Kanagawa, Japan
Abstract Poster Presenter(s)
Yosuke Kunishita1, Kayo Harita2, Chikara Honda2, Masaki Mitsuhasi2 and Shouhei Nagaoka2, 1Department of Rheumatology, Yokohama Minami Kyosai Hospital, Yokohama, Kanagawa, Japan, 2Department of Rheumatology, Yokohama Minami Kyosai Hospital, Yokohama, Japan
Background/Purpose: In 2019, a study based on post-marketing surveillance data in Japan reported that abatacept (ABT) was as effective and safe in elderly patients with rheumatoid arthritis (RA) as non-elderly. However, there were inconsistent reports on the impact of ABT on malignancies which are more common in the elderly and strongly related to prognosis, and there are still unanswered questions. The research objectives were to evaluate the incidence of malignancy, and to evaluate the efficacy and safety with previous malignancy in RA patients using ABT in our clinical practice.
Methods: Patients who received ABT for RA at our department from October 2010 to May 2021 were included in the study. Patient background, prior malignancy at starting ABT, disease activity from starting ABT up to 60 months, continuation rates of ABT, and safety including onset or recurrence of malignancy during ABT use were retrospectively collected from electronic medical records. The patients were divided into two groups according to the absence or presence of previous malignancy, and the collected parameters were compared between the groups.
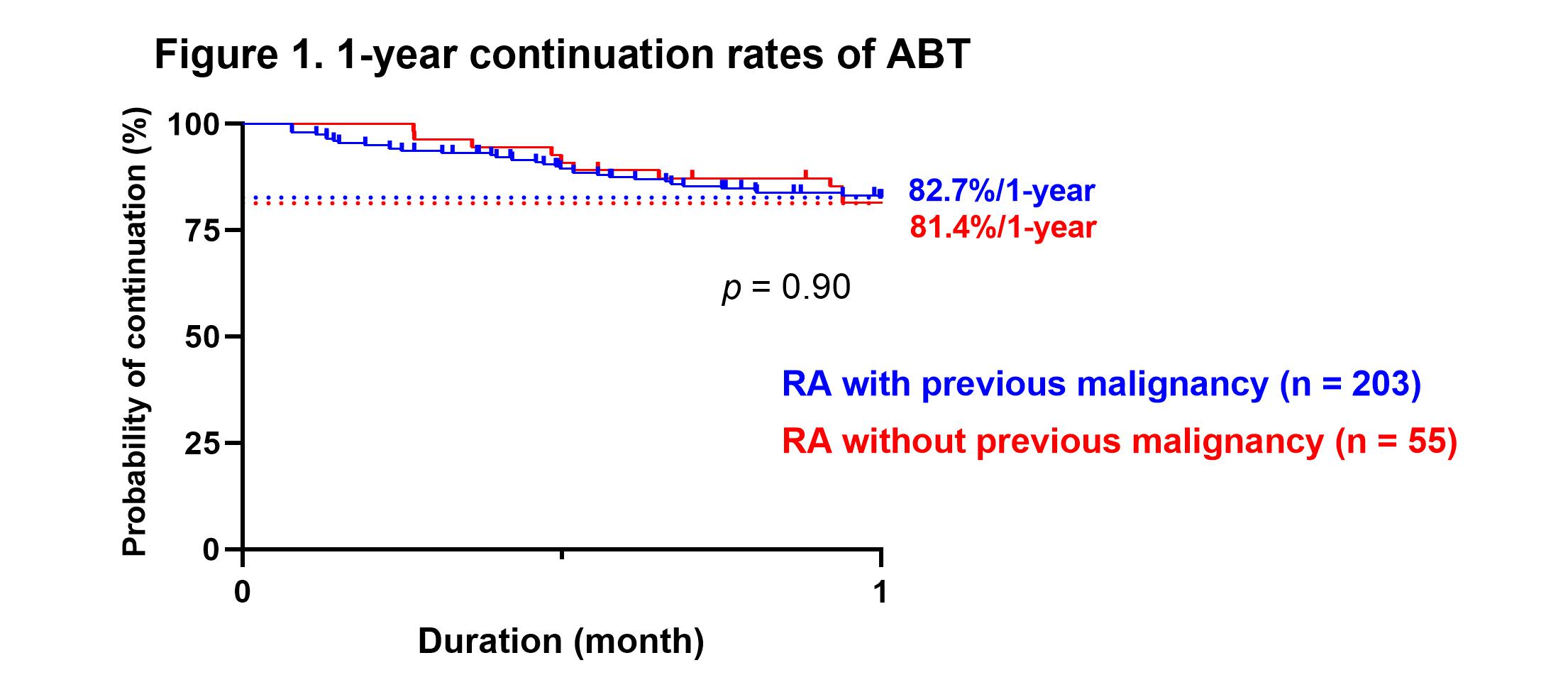
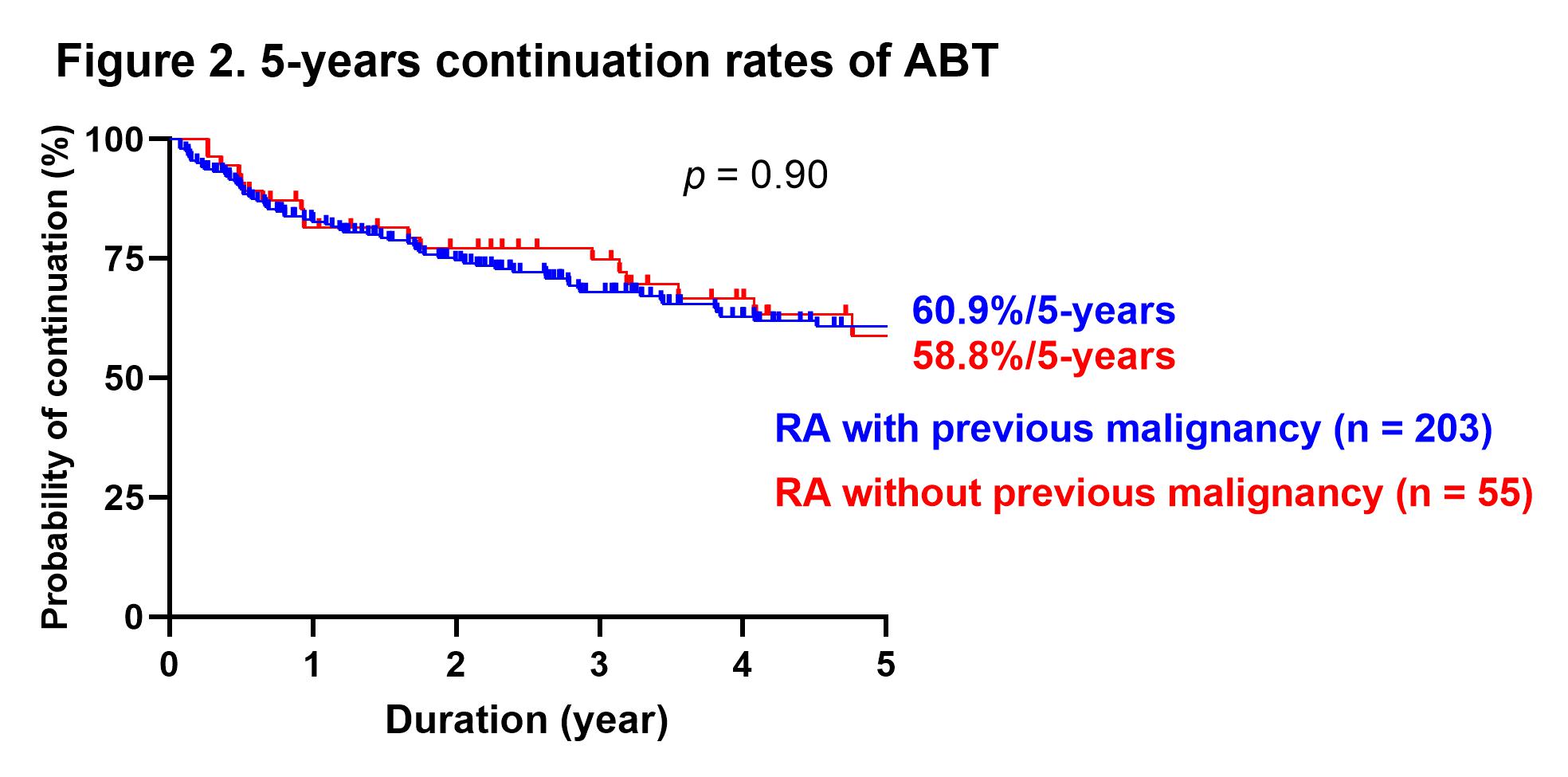
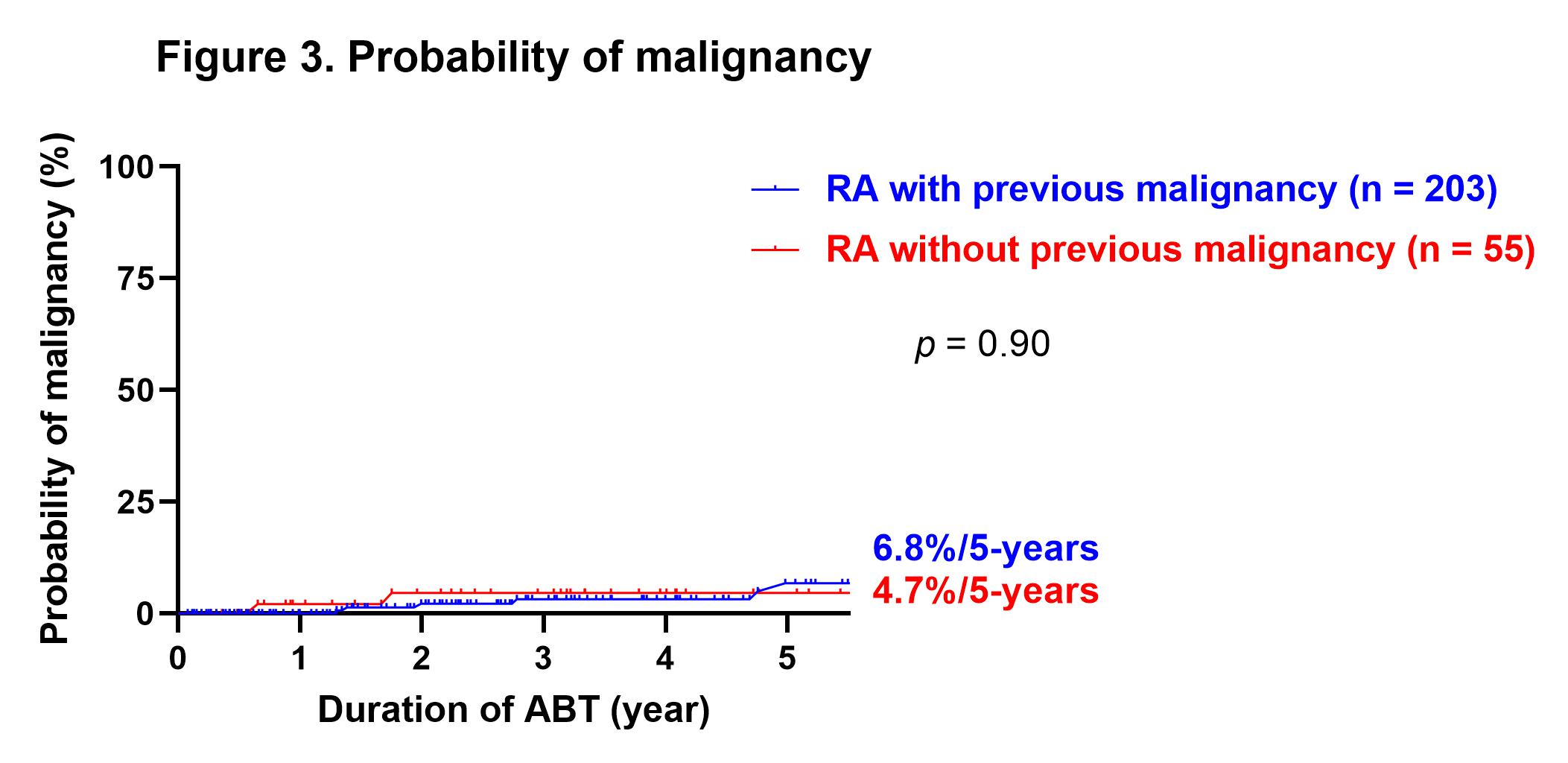
Results: A total of 258 patients were included, of which 55 had previous malignancy at the time of starting ABT. The rate of methotrexate use was significantly lower in the patients with prior malignancy (30.9 vs. 52.7%, p = 0.0041), although there were no significant differences in other patient backgrounds between the two groups. There was no significant difference in the rate of biologic agents and Janus kinase inhibitors naïve patients between patients without or with previous malignancy (72.4 vs. 74.5%). The disease activity improved significantly at three months after starting ABT, and DAS28-CRP remission was maintained after that. There was no significant difference in disease activity between the groups from starting ABT to 60 months after. There were no significant differences for the duration, continuation rates for 1-year and 5-year of ABT between patients without or with previous malignancy (3.5 ± 3.0 vs. 3.5 ± 2.6 years, 82.7 vs. 81.4%, and 60.9 vs. 58.8%, respectively, Figure 1, 2). There was no significant difference for the incidence of relapse or new onset of malignancy at five years after starting ABT between patients without or with previous malignancy (6.8% vs. 4.7%, Figure 3).
Conclusion: In our clinical practice, ABT showed similar efficacy and safety in patients with previous malignancy as in those without.
Disclosures: Y. Kunishita, None; K. Harita, None; C. Honda, None; M. Mitsuhasi, None; S. Nagaoka, None.
Background/Purpose: In 2019, a study based on post-marketing surveillance data in Japan reported that abatacept (ABT) was as effective and safe in elderly patients with rheumatoid arthritis (RA) as non-elderly. However, there were inconsistent reports on the impact of ABT on malignancies which are more common in the elderly and strongly related to prognosis, and there are still unanswered questions. The research objectives were to evaluate the incidence of malignancy, and to evaluate the efficacy and safety with previous malignancy in RA patients using ABT in our clinical practice.
Methods: Patients who received ABT for RA at our department from October 2010 to May 2021 were included in the study. Patient background, prior malignancy at starting ABT, disease activity from starting ABT up to 60 months, continuation rates of ABT, and safety including onset or recurrence of malignancy during ABT use were retrospectively collected from electronic medical records. The patients were divided into two groups according to the absence or presence of previous malignancy, and the collected parameters were compared between the groups.
Results: A total of 258 patients were included, of which 55 had previous malignancy at the time of starting ABT. The rate of methotrexate use was significantly lower in the patients with prior malignancy (30.9 vs. 52.7%, p = 0.0041), although there were no significant differences in other patient backgrounds between the two groups. There was no significant difference in the rate of biologic agents and Janus kinase inhibitors naïve patients between patients without or with previous malignancy (72.4 vs. 74.5%). The disease activity improved significantly at three months after starting ABT, and DAS28-CRP remission was maintained after that. There was no significant difference in disease activity between the groups from starting ABT to 60 months after. There were no significant differences for the duration, continuation rates for 1-year and 5-year of ABT between patients without or with previous malignancy (3.5 ± 3.0 vs. 3.5 ± 2.6 years, 82.7 vs. 81.4%, and 60.9 vs. 58.8%, respectively, Figure 1, 2). There was no significant difference for the incidence of relapse or new onset of malignancy at five years after starting ABT between patients without or with previous malignancy (6.8% vs. 4.7%, Figure 3).
Conclusion: In our clinical practice, ABT showed similar efficacy and safety in patients with previous malignancy as in those without.



Disclosures: Y. Kunishita, None; K. Harita, None; C. Honda, None; M. Mitsuhasi, None; S. Nagaoka, None.

