Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0431: Long-term Follow-up of Starting and Switching from Bio-originator to Biosimilar: Real-world Data in Axial Spondyloarthritis Patients Treated with Adalimumab and Etanercept
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AM
Ana Lúcia Martins Fernandes, MD
Centro Hospitalar Universitário do Algarve
Faro, Portugal
Abstract Poster Presenter(s)
Ana Lúcia Martins Fernandes1, Ana Pinto2, Kalveer flora3, dilpreet matharu3, anthony isaacs3 and Pedro Machado4, 1Centro Hospitalar Universitário do Algarve, Faro, Portugal, 2Local Health Unit of Guarda, Barcelos, Portugal, 3Northwick Park Hospital, London, United Kingdom, 4University College London, London, United Kingdom
Background/Purpose: Biotherapeutics have revolutionized the treatment of axial spondyloarthritis (axSpA). The emergence of biosimilars allowed substantial savings and a wider access to treatment, and international recommendations now contemplate switching strategies from bio-originator (BO) to its biosimilars (BS).
We aim to investigate treatment response to adalimumab (ADA) and etanercept (ETA) in BO- and BS-treated bDMARD-naïve patients with axSpA and in patients who switched from BO to BS respective drug; and to compare the effectiveness and safety of the BO and BS drugs by assessing their persistence rates (PR).
Methods: Retrospective tertiary care observational study that included bDMARD-naïve patients with a diagnosis of axSpA who initiated treatment with ADA/ETA BOs or BSs, and bDMARD-experienced patients who switched from ADA/ETA BO to a BS drug (Hyrimoz/Benepali). Response to treatment was assessed according to UK National Institute for Health and Care Excellence (NICE) guidelines (at least 2/10 units or 50% reduction in BASDAI and 2/10 units reduction in spinal pain) and rates compared at 3 months using the chi-square test. bDMARD PR were calculated using Kaplan-Meier "drug survival curves". Causes of drug discontinuation were summarized using descriptive statistics.
Results: A total of 228 patients were included (154 on ADA and 74 on ETA): regarding ADA, 83 patients were started on BO, 31 on BS, and 40 switched from BO to BS; regarding ETA, 25 patients were started on BO, 33 on BS, and 16 switched from BO to BS. Groups had similar demographic characteristics, apart from the ADA group that switched from BO to BS drug, which had longer disease duration. Regarding baseline clinical characteristics, lower acute phase reactants (CRP, ESR), spinal pain, BASDAI and BASFI scores at baseline were observed in the switch group compared to the bDMARD-naïve groups (except for CRP in the ETA bDMARD-naïve group). Response to treatment after 3 months was similar between originator and biosimilar drugs for both ADA (BO 81.0%; BS 78.3%, p=0.781) and ETA patients (BO: 76.9%, BS: 76.5%, p=0.977).
Three-year PR were similar across groups, both for ADA (figure 1) and ETA (figure 2): ADA PR were 73.5% for BO, 64.5% for BS, and 77.5% for the group that switched from BO to BS (p=0.959); ETA PR were 96.0% for BO, 97.0% for BS and 93.8% on the BO-to-BS switching group (p=0.449). Adverse events and inefficacy were the most frequent causes of discontinuation in ADA patients, and inefficacy and other reasons (e.g., fear of SARS-CoV-2 infection) were the most frequent causes of discontinuation in ETA patients.
Conclusion: First-line treatment with bio-originator or biosimilar ADA/ETA showed similar effectiveness and safety in our long-term cohort of patients with axSpA. Response to treatment was also similar between groups.
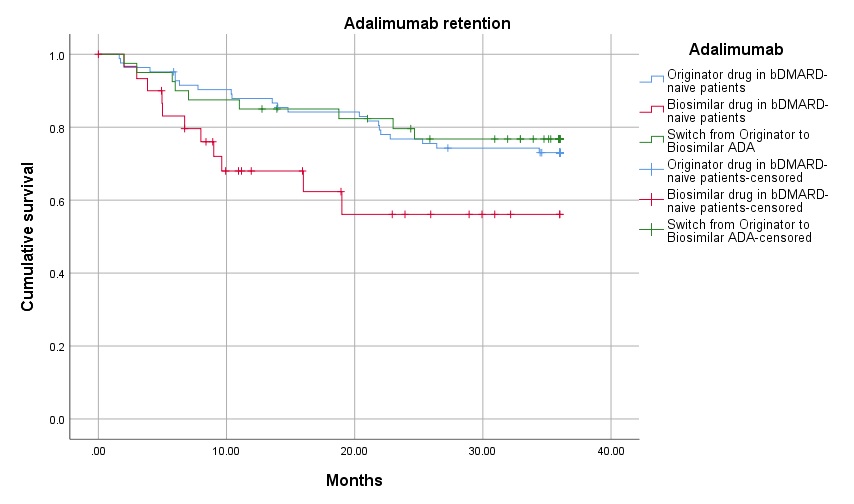 Drug survival in the three adalimumab groups (originator adalimumab – blue line, biosimilar adalimumab – red line, and switch from originator to biosimilar adalimumab – green line)
Drug survival in the three adalimumab groups (originator adalimumab – blue line, biosimilar adalimumab – red line, and switch from originator to biosimilar adalimumab – green line)
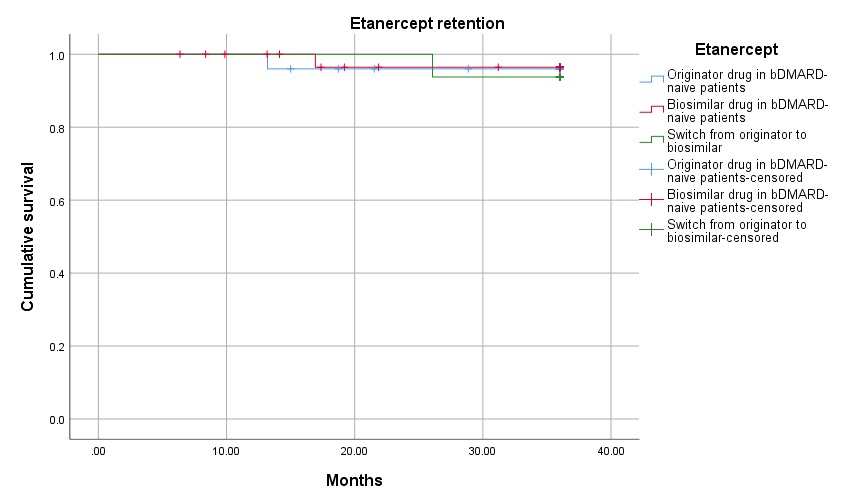 Drug survival in the three etanercept groups (originator etanercept – blue line, biosimilar etanercept – red line, and switch from originator to biosimilar etanercept – green line)
Drug survival in the three etanercept groups (originator etanercept – blue line, biosimilar etanercept – red line, and switch from originator to biosimilar etanercept – green line)
Disclosures: A. Martins Fernandes, None; A. Pinto, None; K. flora, None; d. matharu, None; a. isaacs, None; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos.
Background/Purpose: Biotherapeutics have revolutionized the treatment of axial spondyloarthritis (axSpA). The emergence of biosimilars allowed substantial savings and a wider access to treatment, and international recommendations now contemplate switching strategies from bio-originator (BO) to its biosimilars (BS).
We aim to investigate treatment response to adalimumab (ADA) and etanercept (ETA) in BO- and BS-treated bDMARD-naïve patients with axSpA and in patients who switched from BO to BS respective drug; and to compare the effectiveness and safety of the BO and BS drugs by assessing their persistence rates (PR).
Methods: Retrospective tertiary care observational study that included bDMARD-naïve patients with a diagnosis of axSpA who initiated treatment with ADA/ETA BOs or BSs, and bDMARD-experienced patients who switched from ADA/ETA BO to a BS drug (Hyrimoz/Benepali). Response to treatment was assessed according to UK National Institute for Health and Care Excellence (NICE) guidelines (at least 2/10 units or 50% reduction in BASDAI and 2/10 units reduction in spinal pain) and rates compared at 3 months using the chi-square test. bDMARD PR were calculated using Kaplan-Meier "drug survival curves". Causes of drug discontinuation were summarized using descriptive statistics.
Results: A total of 228 patients were included (154 on ADA and 74 on ETA): regarding ADA, 83 patients were started on BO, 31 on BS, and 40 switched from BO to BS; regarding ETA, 25 patients were started on BO, 33 on BS, and 16 switched from BO to BS. Groups had similar demographic characteristics, apart from the ADA group that switched from BO to BS drug, which had longer disease duration. Regarding baseline clinical characteristics, lower acute phase reactants (CRP, ESR), spinal pain, BASDAI and BASFI scores at baseline were observed in the switch group compared to the bDMARD-naïve groups (except for CRP in the ETA bDMARD-naïve group). Response to treatment after 3 months was similar between originator and biosimilar drugs for both ADA (BO 81.0%; BS 78.3%, p=0.781) and ETA patients (BO: 76.9%, BS: 76.5%, p=0.977).
Three-year PR were similar across groups, both for ADA (figure 1) and ETA (figure 2): ADA PR were 73.5% for BO, 64.5% for BS, and 77.5% for the group that switched from BO to BS (p=0.959); ETA PR were 96.0% for BO, 97.0% for BS and 93.8% on the BO-to-BS switching group (p=0.449). Adverse events and inefficacy were the most frequent causes of discontinuation in ADA patients, and inefficacy and other reasons (e.g., fear of SARS-CoV-2 infection) were the most frequent causes of discontinuation in ETA patients.
Conclusion: First-line treatment with bio-originator or biosimilar ADA/ETA showed similar effectiveness and safety in our long-term cohort of patients with axSpA. Response to treatment was also similar between groups.
 Drug survival in the three adalimumab groups (originator adalimumab – blue line, biosimilar adalimumab – red line, and switch from originator to biosimilar adalimumab – green line)
Drug survival in the three adalimumab groups (originator adalimumab – blue line, biosimilar adalimumab – red line, and switch from originator to biosimilar adalimumab – green line) Drug survival in the three etanercept groups (originator etanercept – blue line, biosimilar etanercept – red line, and switch from originator to biosimilar etanercept – green line)
Drug survival in the three etanercept groups (originator etanercept – blue line, biosimilar etanercept – red line, and switch from originator to biosimilar etanercept – green line)Disclosures: A. Martins Fernandes, None; A. Pinto, None; K. flora, None; d. matharu, None; a. isaacs, None; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos.

