Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0293: Long-term Sustainability of Response to Upadacitinib Among Patients with Active Rheumatoid Arthritis Refractory to Biological Disease-Modifying Anti-Rheumatic Drugs: Results Through 5 Years from SELECT-BEYOND
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- RV
Ronald F. Van Vollenhoven, MD, PhD
Amsterdam UMC
Amsterdam, Netherlands
Abstract Poster Presenter(s)
Ronald van Vollenhoven1, Stephen Hall2, Alvin Wells3, Sebastian Meerwein4, Yanna Song5, Jessica Suboticki6 and Roy Fleischmann7, 1Amsterdam University Medical Centers, Amsterdam, Netherlands, 2Emeritus Research and Monash University, Melbourne, Australia, 3Aurora Rheumatology and Immunotherapy Center, Franklin, WI, 4AbbVie Deutschland GmbH & Co KG, LUDWIGSHAFEN, Germany, 5AbbVie, Inc., North Chicago, IL, 6AbbVie, Inc., Mettawa, IL, 7Metroplex Clinical Research Center and University of Texas Southwestern Medical Center, Dallas, TX
Background/Purpose: The primary treatment goal for patients with rheumatoid arthritis (RA) is sustained remission (REM), but low disease activity (LDA) is considered an appropriate target for those who cannot achieve REM; patients with prior inadequate response or intolerance to biological DMARD therapy (bDMARD-IR) may belong to this group. The objective of this analysis was to evaluate the sustainability of response to the JAK inhibitor upadacitinib (UPA) over 5 years among bDMARD-IR patients.
Methods: Data are from SELECT-BEYOND, a phase 3 randomized trial of UPA 15 mg or 30 mg once daily (placebo [PBO]-controlled for the first 12 weeks) in patients with RA on background conventional synthetic (cs) DMARDs.1 Patients who completed the week 24 visit could enter a long-term extension of up to a total of 5 years. Initiation, change, or discontinuation of background RA medications, including ≤2 csDMARDs, was permitted starting at week 24 per investigators' discretion. This post hoc analysis only includes data from patients receiving the approved 15 mg dosage: both those randomized to UPA 15 mg and those who switched from PBO to UPA 15 mg. The proportions of patients who achieved REM (CDAI ≤2.8; SDAI ≤3.3), LDA (CDAI ≤10; SDAI ≤11), and DAS28(CRP) < 2.6/≤3.2 between the first dose of UPA and the end of study were assessed. Sustained response was evaluated using the Kaplan-Meier method and defined as the time from when the response was first achieved while on UPA to the earliest date at which the response was lost at two consecutive visits or discontinuation of study drug due to lack of efficacy. Recapture of response was assessed in patients who lost response at 2 consecutive visits but later regained response. The predictive ability of key baseline and demographic characteristics was determined using Harrell's concordance-index. All analyses used observed case, with discontinuation of the study drug due to lack of efficacy treated as a loss of response.
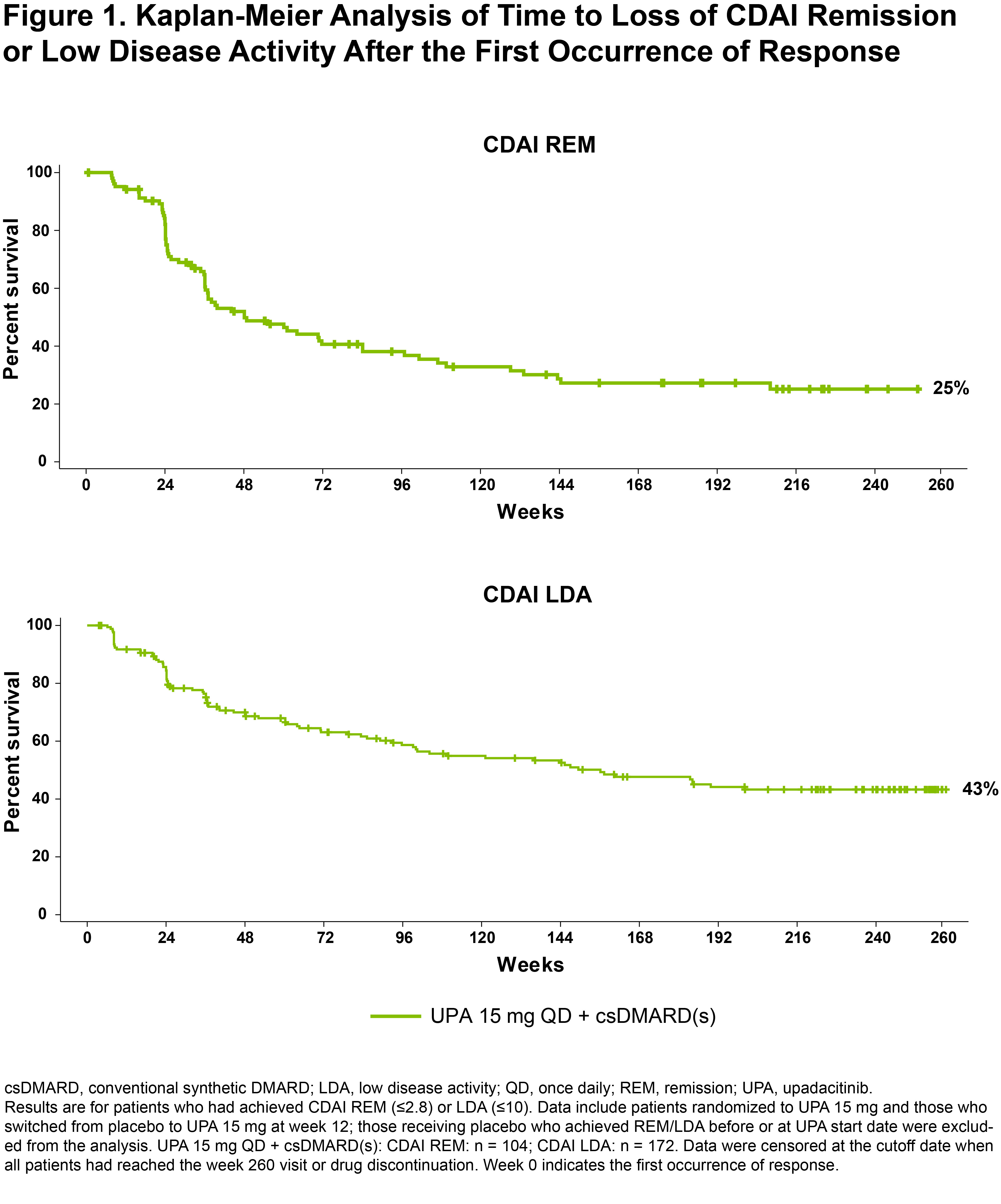
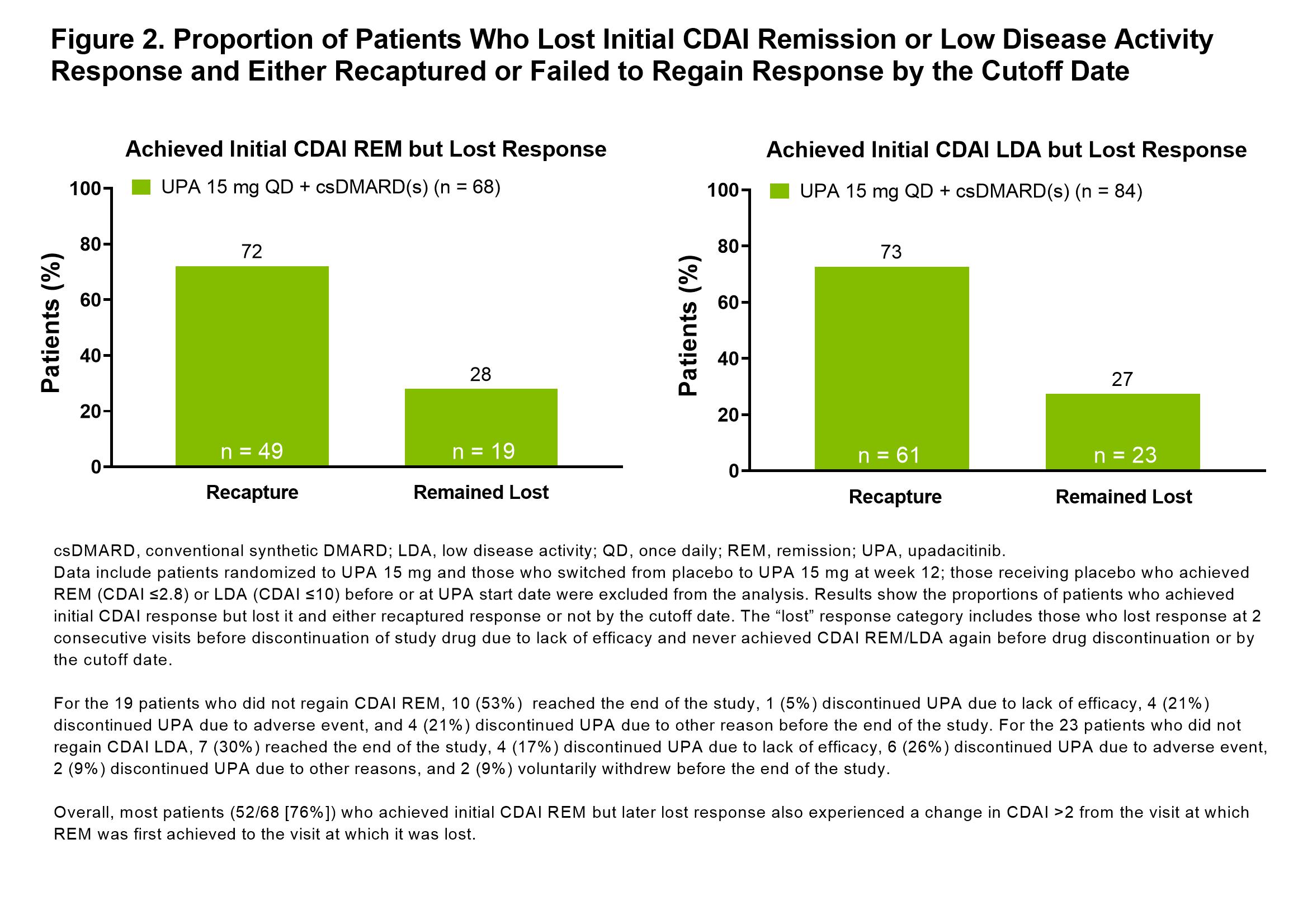
Results: In this bDMARD-IR population, 104/229 (45%) and 172/219 (79%) of those receiving UPA 15 mg + background csDMARD(s) achieved CDAI REM or LDA at any point during the 5-year study, respectively. At weeks 12, 24, and 48, 10%/37%, 17%/55%, and 29%/70% of patients achieved CDAI REM/LDA. Through the last follow-up visit, 25%/43% of patients receiving UPA sustained CDAI REM/LDA response (Figure 1). The median duration of response was 48 weeks for CDAI REM and 156 weeks for CDAI LDA. Most patients who had lost CDAI REM or CDAI LDA were able to recapture response by the cutoff date (72% [49/68] recaptured CDAI REM and 73% [61/84] recaptured LDA) (Figure 2). No strong predictors of CDAI REM/LDA were identified. Similar overall results were observed for SDAI REM/LDA and for DAS28(CRP) < 2.6/≤3.2.
Conclusion: Most bDMARD-IR patients achieved CDAI LDA on UPA and around half of those maintained LDA through the end of study. Remission was achieved less frequently and maintained in a quarter of the patients. These data underscore that LDA may be a reasonable treatment target in bDMARD-IR patients and that UPA may help achieve that goal in such patients.
References:
1. Genovese, MC et al. Lancet 2018;391:2513–24.
Disclosures: R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; S. Hall, AbbVie, Bristol-Myers Squibb (BMS), Lilly, Janssen, UCB, Novartis, Amgen, Gilead, Merck; A. Wells, AbbVie; S. Meerwein, AbbVie; Y. Song, AbbVie; J. Suboticki, AbbVie; R. Fleischmann, AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Galvani, GlaxoSmithKlein (GSK), Janssen, Pfizer, Gilead, UCB, Novartis, Arthrosi, AstraZeneca, Flexion, Genentech, Horizon, Selecta, Viela, Vorso, Vyne.
Background/Purpose: The primary treatment goal for patients with rheumatoid arthritis (RA) is sustained remission (REM), but low disease activity (LDA) is considered an appropriate target for those who cannot achieve REM; patients with prior inadequate response or intolerance to biological DMARD therapy (bDMARD-IR) may belong to this group. The objective of this analysis was to evaluate the sustainability of response to the JAK inhibitor upadacitinib (UPA) over 5 years among bDMARD-IR patients.
Methods: Data are from SELECT-BEYOND, a phase 3 randomized trial of UPA 15 mg or 30 mg once daily (placebo [PBO]-controlled for the first 12 weeks) in patients with RA on background conventional synthetic (cs) DMARDs.1 Patients who completed the week 24 visit could enter a long-term extension of up to a total of 5 years. Initiation, change, or discontinuation of background RA medications, including ≤2 csDMARDs, was permitted starting at week 24 per investigators' discretion. This post hoc analysis only includes data from patients receiving the approved 15 mg dosage: both those randomized to UPA 15 mg and those who switched from PBO to UPA 15 mg. The proportions of patients who achieved REM (CDAI ≤2.8; SDAI ≤3.3), LDA (CDAI ≤10; SDAI ≤11), and DAS28(CRP) < 2.6/≤3.2 between the first dose of UPA and the end of study were assessed. Sustained response was evaluated using the Kaplan-Meier method and defined as the time from when the response was first achieved while on UPA to the earliest date at which the response was lost at two consecutive visits or discontinuation of study drug due to lack of efficacy. Recapture of response was assessed in patients who lost response at 2 consecutive visits but later regained response. The predictive ability of key baseline and demographic characteristics was determined using Harrell's concordance-index. All analyses used observed case, with discontinuation of the study drug due to lack of efficacy treated as a loss of response.
Results: In this bDMARD-IR population, 104/229 (45%) and 172/219 (79%) of those receiving UPA 15 mg + background csDMARD(s) achieved CDAI REM or LDA at any point during the 5-year study, respectively. At weeks 12, 24, and 48, 10%/37%, 17%/55%, and 29%/70% of patients achieved CDAI REM/LDA. Through the last follow-up visit, 25%/43% of patients receiving UPA sustained CDAI REM/LDA response (Figure 1). The median duration of response was 48 weeks for CDAI REM and 156 weeks for CDAI LDA. Most patients who had lost CDAI REM or CDAI LDA were able to recapture response by the cutoff date (72% [49/68] recaptured CDAI REM and 73% [61/84] recaptured LDA) (Figure 2). No strong predictors of CDAI REM/LDA were identified. Similar overall results were observed for SDAI REM/LDA and for DAS28(CRP) < 2.6/≤3.2.
Conclusion: Most bDMARD-IR patients achieved CDAI LDA on UPA and around half of those maintained LDA through the end of study. Remission was achieved less frequently and maintained in a quarter of the patients. These data underscore that LDA may be a reasonable treatment target in bDMARD-IR patients and that UPA may help achieve that goal in such patients.
References:
1. Genovese, MC et al. Lancet 2018;391:2513–24.


Disclosures: R. van Vollenhoven, Bristol Myers Squibb (BMS), GlaxoSmithKline (GSK), UCB, Merck/MSD, Pfizer, Roche, AbbVie, AstraZeneca, Biogen, Galapagos, Janssen, Miltenyi, R-Pharma; S. Hall, AbbVie, Bristol-Myers Squibb (BMS), Lilly, Janssen, UCB, Novartis, Amgen, Gilead, Merck; A. Wells, AbbVie; S. Meerwein, AbbVie; Y. Song, AbbVie; J. Suboticki, AbbVie; R. Fleischmann, AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly, Galvani, GlaxoSmithKlein (GSK), Janssen, Pfizer, Gilead, UCB, Novartis, Arthrosi, AstraZeneca, Flexion, Genentech, Horizon, Selecta, Viela, Vorso, Vyne.

