Back
Poster Session A
Systemic lupus erythematosus (SLE)
Session: (0317–0342) SLE – Diagnosis, Manifestations, and Outcomes Poster I: Diagnosis
0328: Predicting Progression from Undifferentiated to Definitive Connective Tissue Diseases: A Systematic Review and Meta-analysis
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SD
Sarah Dyball, MBBS, BSc
University of Manchester
Manchester, United Kingdom
Abstract Poster Presenter(s)
Sarah Dyball1, Mia Rodziewicz1, Claudia Mendoza Pinto2, Ian N. Bruce3 and Ben Parker4, 1The University of Manchester, Manchester, United Kingdom, 2Systemic Autoimmune Diseases Research Unit, High-Specialty Medical Unit-CIBIOR, Mexican Social Security Institute, Puebla, Mexico, 3Centre for Epidemiology Versus Arthritis, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester, United Kingdom, 4Manchester University Hospitals NHS Foundation Trust, Manchester, United Kingdom
Background/Purpose: Undifferentiated connective tissue disease (UCTD) encapsulates a broad range of conditions including incomplete forms of systemic lupus erythematosus (SLE) and systemic sclerosis (SSc), some of whom progress to a formal clinical diagnosis over time. It is characterised by clinical and laboratory features suggestive of a systemic autoimmune disease that are not sufficient to diagnose a defined connective tissue disease (CTD). This systematic review and meta-analysis aimed to identify clinical and laboratory features and biomarkers that can predict progression of UCTD.
Methods: MEDLINE, EMBASE and the Cochrane Central Register of Randomized Controlled Trials were systematically searched from inception until February 2021. Abstracts and full-text manuscripts were screened by two reviewers. Publications were included if they included at least 20 UCTD patients, a minimum of six months of follow up, and provided data on at least one risk factor for developing a defined CTD. QUIPS tool was used to assess risk of bias and GRADE approach for grading the quality of the evidence. For predictors reported in at least two studies, meta-analysis was carried out using random-effects models to pool effect sizes. Heterogeneity was assessed using the standard chi-squared test and I2 statistic. Influence analysis was carried out to identify outlier studies with extreme effect sizes. Publication bias was assessed by visual inspection of funnel plots and Egger's test. The study is registered with PROSPERO (ID: CRD42021237725)
Results: A total of 3871 articles were initially identified via the literature search; 2559 abstracts were screened and 196 full-texts were reviewed for eligibility. Forty-five studies were included in the systematic review, and thirty-three in the meta-analysis. The predictors for progression to SLE with the highest quality of evidence included those with younger age, serositis, or the presence of anti-dsDNA antibodies (table 1). For SSc, the highest quality of evidence included puffy fingers, abnormal nailfold changes (avascular areas or giant capillaries) and SSc specific antibodies (table 2). No novel biomarkers were included in the meta-analysis however HLA antigens, T-regulatory cell shift, and complement activation products were reported as potential predictors for SLE. All studies were rated as having a high or moderate risk of bias (figure 1).
Conclusion: Clinical and immunological parameters may predict which patients with UCTD progress to definitive disease however, the heterogeneous nature and risk of bias in most studies limits the ability to apply these results in routine clinical practice. Limited data suggest that some novel biomarkers may provide additional predictive value but these will need larger well designed studies to fully delineate their clinical utility.
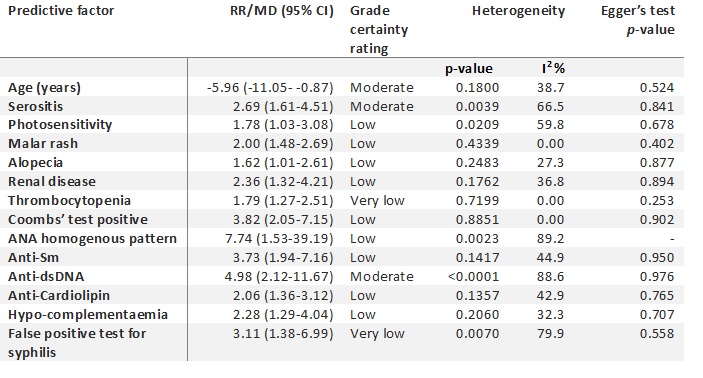 Table 1 Predictors for progression from UCTD to SLE. ANA, antinuclear antigen; anti-dsDNA, anti-double stranded DNA; anti-Sm, anti-Smith; GRADE, grading of recommendations assessment development and evaluations; MD, mean difference; RR, relative risk; SLE, systemic lupus erythematosus; UCTD, undifferentiated connective tissue disease
Table 1 Predictors for progression from UCTD to SLE. ANA, antinuclear antigen; anti-dsDNA, anti-double stranded DNA; anti-Sm, anti-Smith; GRADE, grading of recommendations assessment development and evaluations; MD, mean difference; RR, relative risk; SLE, systemic lupus erythematosus; UCTD, undifferentiated connective tissue disease
.jpg) Table 2 Predictors for progression from UCTD to SSc. ANA, antinuclear antigen; GRADE, grading of recommendations assessment development and evaluations; MD, mean difference; NFC, nail-fold capillaroscopy; RR, relative risk; SSc, systemic sclerosis; UCTD, undifferentiated connective tissue disease
Table 2 Predictors for progression from UCTD to SSc. ANA, antinuclear antigen; GRADE, grading of recommendations assessment development and evaluations; MD, mean difference; NFC, nail-fold capillaroscopy; RR, relative risk; SSc, systemic sclerosis; UCTD, undifferentiated connective tissue disease
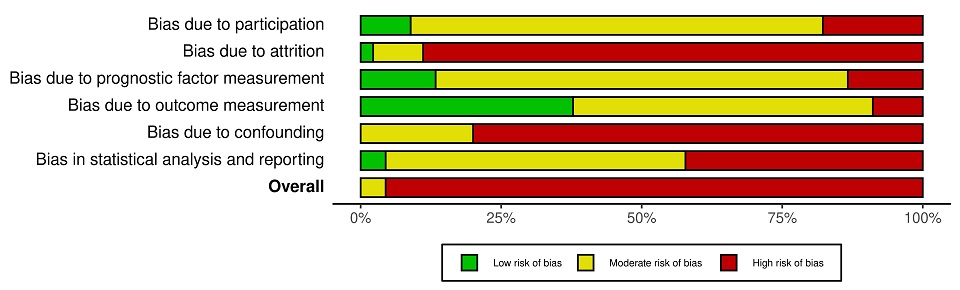 Figure 1 Quality in prognostic studies (QUIPS) analysis across studies (n=45) included in the systematic review
Figure 1 Quality in prognostic studies (QUIPS) analysis across studies (n=45) included in the systematic review
Disclosures: S. Dyball, UCB, Eli Lilly; M. Rodziewicz, UCB; C. Mendoza Pinto, None; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); B. Parker, None.
Background/Purpose: Undifferentiated connective tissue disease (UCTD) encapsulates a broad range of conditions including incomplete forms of systemic lupus erythematosus (SLE) and systemic sclerosis (SSc), some of whom progress to a formal clinical diagnosis over time. It is characterised by clinical and laboratory features suggestive of a systemic autoimmune disease that are not sufficient to diagnose a defined connective tissue disease (CTD). This systematic review and meta-analysis aimed to identify clinical and laboratory features and biomarkers that can predict progression of UCTD.
Methods: MEDLINE, EMBASE and the Cochrane Central Register of Randomized Controlled Trials were systematically searched from inception until February 2021. Abstracts and full-text manuscripts were screened by two reviewers. Publications were included if they included at least 20 UCTD patients, a minimum of six months of follow up, and provided data on at least one risk factor for developing a defined CTD. QUIPS tool was used to assess risk of bias and GRADE approach for grading the quality of the evidence. For predictors reported in at least two studies, meta-analysis was carried out using random-effects models to pool effect sizes. Heterogeneity was assessed using the standard chi-squared test and I2 statistic. Influence analysis was carried out to identify outlier studies with extreme effect sizes. Publication bias was assessed by visual inspection of funnel plots and Egger's test. The study is registered with PROSPERO (ID: CRD42021237725)
Results: A total of 3871 articles were initially identified via the literature search; 2559 abstracts were screened and 196 full-texts were reviewed for eligibility. Forty-five studies were included in the systematic review, and thirty-three in the meta-analysis. The predictors for progression to SLE with the highest quality of evidence included those with younger age, serositis, or the presence of anti-dsDNA antibodies (table 1). For SSc, the highest quality of evidence included puffy fingers, abnormal nailfold changes (avascular areas or giant capillaries) and SSc specific antibodies (table 2). No novel biomarkers were included in the meta-analysis however HLA antigens, T-regulatory cell shift, and complement activation products were reported as potential predictors for SLE. All studies were rated as having a high or moderate risk of bias (figure 1).
Conclusion: Clinical and immunological parameters may predict which patients with UCTD progress to definitive disease however, the heterogeneous nature and risk of bias in most studies limits the ability to apply these results in routine clinical practice. Limited data suggest that some novel biomarkers may provide additional predictive value but these will need larger well designed studies to fully delineate their clinical utility.
 Table 1 Predictors for progression from UCTD to SLE. ANA, antinuclear antigen; anti-dsDNA, anti-double stranded DNA; anti-Sm, anti-Smith; GRADE, grading of recommendations assessment development and evaluations; MD, mean difference; RR, relative risk; SLE, systemic lupus erythematosus; UCTD, undifferentiated connective tissue disease
Table 1 Predictors for progression from UCTD to SLE. ANA, antinuclear antigen; anti-dsDNA, anti-double stranded DNA; anti-Sm, anti-Smith; GRADE, grading of recommendations assessment development and evaluations; MD, mean difference; RR, relative risk; SLE, systemic lupus erythematosus; UCTD, undifferentiated connective tissue disease.jpg) Table 2 Predictors for progression from UCTD to SSc. ANA, antinuclear antigen; GRADE, grading of recommendations assessment development and evaluations; MD, mean difference; NFC, nail-fold capillaroscopy; RR, relative risk; SSc, systemic sclerosis; UCTD, undifferentiated connective tissue disease
Table 2 Predictors for progression from UCTD to SSc. ANA, antinuclear antigen; GRADE, grading of recommendations assessment development and evaluations; MD, mean difference; NFC, nail-fold capillaroscopy; RR, relative risk; SSc, systemic sclerosis; UCTD, undifferentiated connective tissue disease Figure 1 Quality in prognostic studies (QUIPS) analysis across studies (n=45) included in the systematic review
Figure 1 Quality in prognostic studies (QUIPS) analysis across studies (n=45) included in the systematic reviewDisclosures: S. Dyball, UCB, Eli Lilly; M. Rodziewicz, UCB; C. Mendoza Pinto, None; I. Bruce, AstraZeneca, Bristol-Myers Squibb(BMS), Eli Lilly, Aurinia, Janssen, GlaxoSmithKlein(GSK); B. Parker, None.

