Back
Poster Session A
Rheumatoid arthritis (RA)
Session: (0272–0316) RA – Treatment Poster I
0295: Safety and Efficacy of Upadacitinib in Patients with Rheumatoid Arthritis and Inadequate Response to Conventional Synthetic DMARDs: Results Through 5 Years from the SELECT-NEXT Study
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- GB
Gerd Burmester, MD
EULAR
Berlin, Germany
Abstract Poster Presenter(s)
Gerd Burmester1, Filip Van den bosch2, John Tesser3, Anna K Shmagel4, Yuanyuan Duan4, Nasser Khan5, Heidi Camp6 and Alan Kivitz7, 1Charité University Medicine Berlin, Berlin, Germany, 2Department of Internal Medicine and Paediatrics, Ghent University and VIB Centre for Inflammation Research, Ghent, Belgium, 3Arizona Arthritis & Rheumatology Associates, Phoenix, AZ, 4AbbVie, Inc., North Chicago, IL, 5AbbVie, Inc., Abbott Park, IL, 6Abbvie, Winnetka, IL, 7Department of Rheumatology, Altoona Center for Clinical Research, Duncansville, PA
Background/Purpose: In the phase 3 SELECT-NEXT study, upadacitinib (UPA) demonstrated efficacy at wk 12 and sustained efficacy up to wk 60 in patients with rheumatoid arthritis (RA) and inadequate response to conventional synthetic DMARDs (csDMARDs).1,2 Here, we evaluated the efficacy and safety of UPA up to 5 yrs in a long-term extension (LTE) of SELECT-NEXT.
Methods: Patients received UPA 15 mg (UPA15) or 30 mg (UPA30) once daily or placebo (PBO) for 12 wks, each with stable background csDMARDs. Patients randomized to PBO were switched to UPA15/30 in a pre-specified manner at wk 12. From wk 12 onwards, patients were able to enter a blinded LTE for up to a total of 5 yrs, in which all patients received UPA15 or UPA30, and both the study site and patients were blinded to the UPA dose administered. The blinded extension remained until dose switching from UPA30 to UPA15, per protocol amendment, with the earliest switch occurring at wk 168. For patients not meeting CDAI ≤10 at or after wk 24, investigators were instructed to adjust background RA therapies. Efficacy data up to wk 260 are reported as observed (AO) for patients randomized to UPA15 or UPA30. Missing data for categorical endpoints were also imputed using NRI. Treatment-emergent adverse events (TEAEs) per 100 patient yrs (PY) were summarized over 5 yrs.
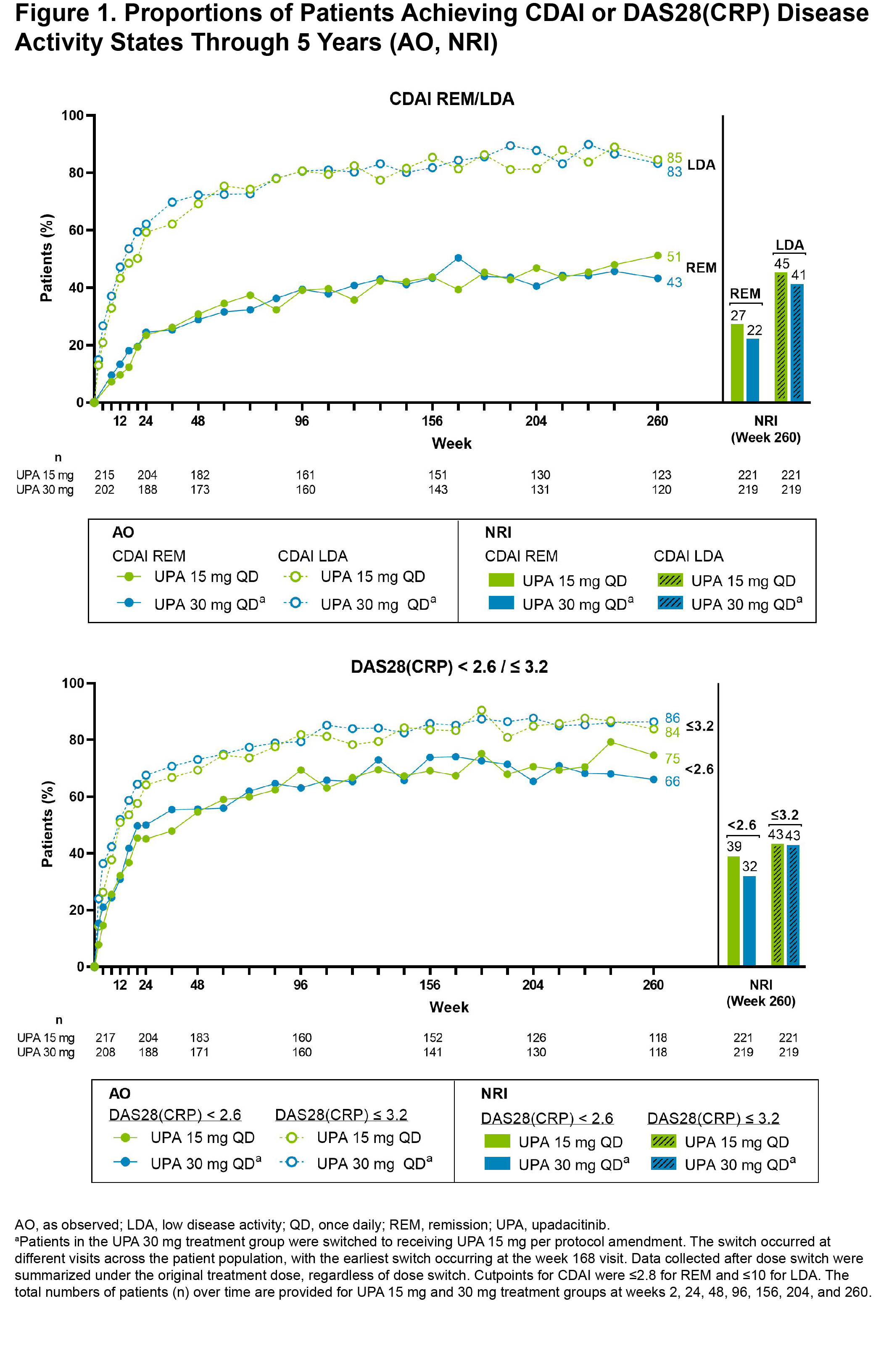
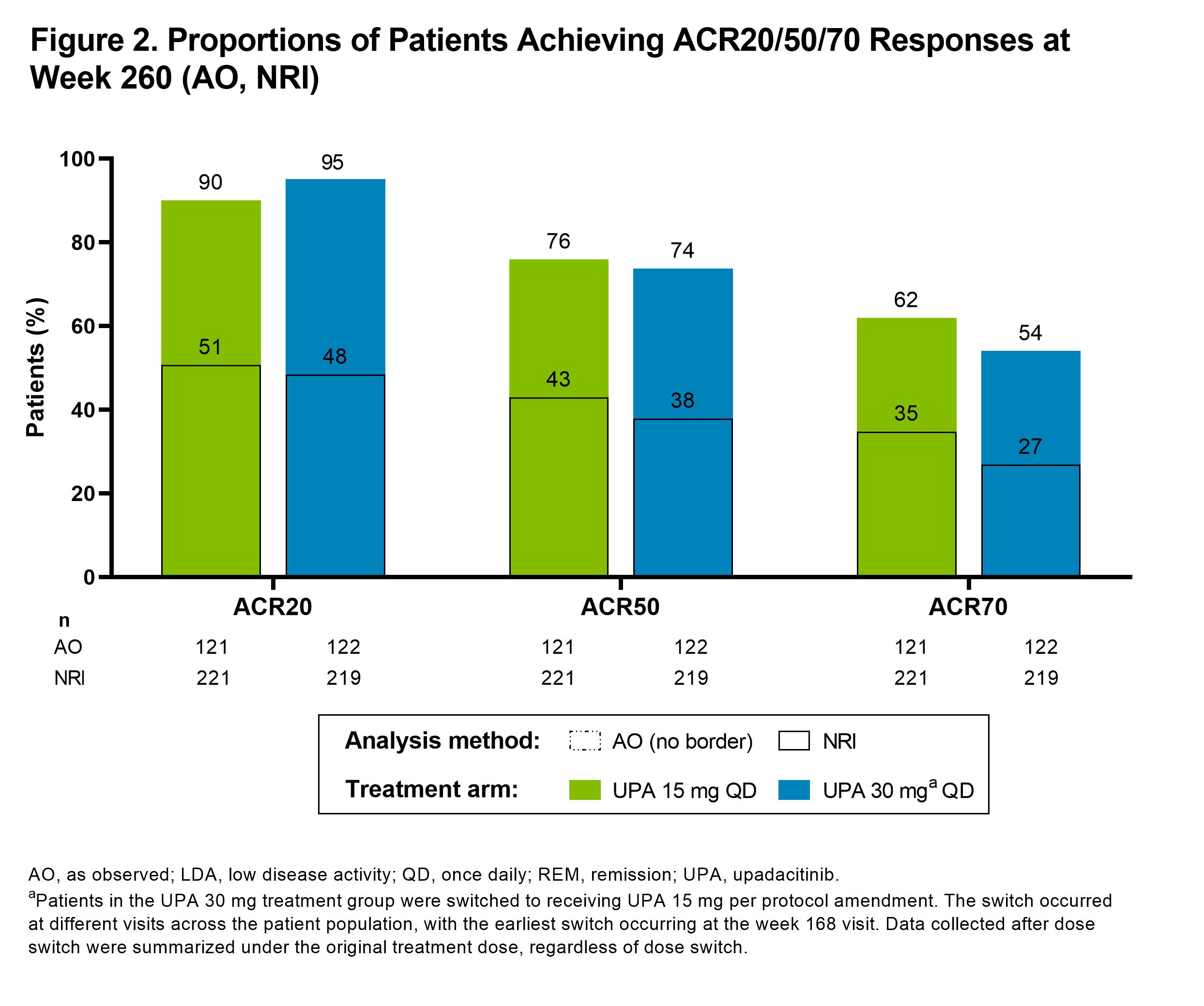
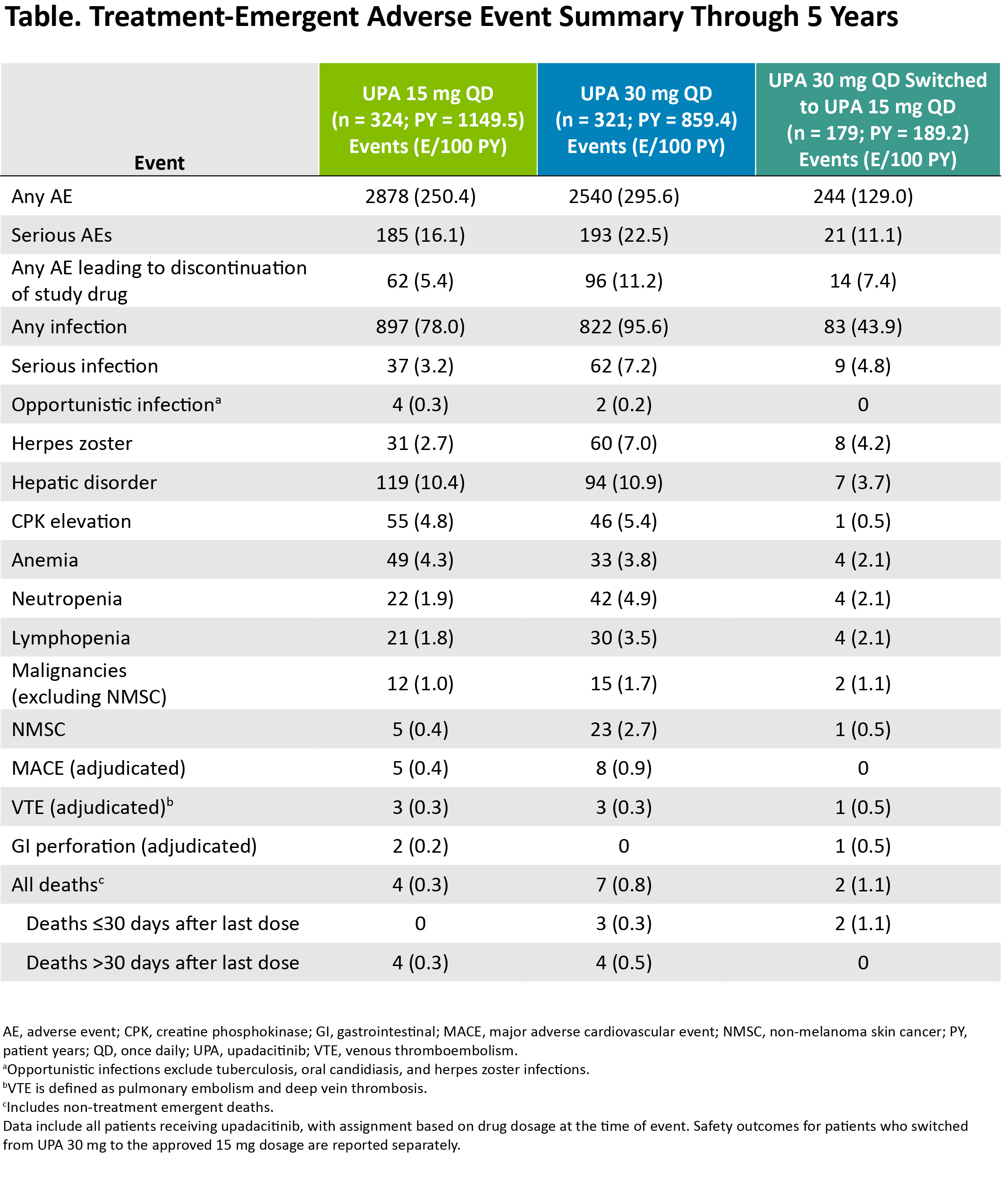
Results: Of the 661 patients randomized at baseline, 611 (92%) completed wk 12 and entered the 248-wk LTE. In total, 271 (41%) patients discontinued study drug during the LTE due to adverse events (16%), withdrawal of consent (10%), loss to follow-up (3%), lack of efficacy (3%), or other reasons (9%). Clinical outcomes continued to improve or were maintained through wk 260 (Figure 1), as demonstrated by 51% and 43% of patients achieving CDAI remission and 75% and 66% attaining DAS28(CRP) < 2.6 with UPA15 and UPA30, respectively (AO). ACR20/50/70 responses at wk 260 are shown in Figure 2, with over half of patients achieving ACR70 on either UPA dose (AO). Lower efficacy estimates based on NRI are also reported and were consistent for both dosage groups. Physical function and pain improved similarly with UPA15/30 at wk 260; the mean change from baseline was -0.86/-0.78 for HAQ-DI and -44/-44 mm for pain (AO). Patients who switched from PBO to UPA at wk 12 showed similar efficacy as those who were initiated on UPA (data not shown). Through 5 yrs, TEAEs and AEs of special interest were consistent with previous study-specific analyses (Table).2 Numerically higher rates of any AE, serious AEs, any infection, serious infections, herpes zoster, NMSC, and neutropenia were observed with UPA30 vs UPA15. While malignancies (excluding NMSC) and MACE were rare, numerically higher rates were also observed with UPA30 compared to UPA15.
Conclusion: UPA 15 mg or 30 mg therapy with background csDMARDs demonstrated sustained efficacy across multiple RA disease activity measures through the 5-yr study period. The safety profile was consistent with earlier time points and with an integrated phase 3 safety analysis of UPA in RA,3 although dose-dependent increases in the rates of certain adverse events were noted in patients receiving UPA30 vs UPA15.
1. Burmester, et al. Lancet 2018;391:2503–12
2. Burmester, et al. Ann Rheum Dis 2019;78:735–6
3. Cohen, et al. Ann Rheum Dis 2021: 80:304–11
Disclosures: G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc.; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene; J. Tesser, AbbVie, Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, GlaxoSmithKlein (GSK), Janssen, Gilead, Merck/MSD, Novartis, Pfizer, UCB, Aurinia, Genentech, Sanofi-Genzyme, Biogen, Celgene, CSL Behring, Horizon, R-Pharma, Regeneron, Roche, Sandoz, Scipher, Takeda, Sun Pharma, Selecta, Vorso; A. Shmagel, AbbVie; Y. Duan, AbbVie; N. Khan, AbbVie; H. Camp, AbbVie; A. Kivitz, Amgen, Boehringer-Ingelheim, Janssen, Gilead, GlaxoSmithKlein (GSK), Novartis, Pfizer, Sanofi, Flexion, Eli Lilly, Genentech, UCB, AbbVie, Merck, ECOR1 CAPITAL, LLC, Chemocentryx, Regenerson, Grunenthal, Bendcare, Horizon.
Background/Purpose: In the phase 3 SELECT-NEXT study, upadacitinib (UPA) demonstrated efficacy at wk 12 and sustained efficacy up to wk 60 in patients with rheumatoid arthritis (RA) and inadequate response to conventional synthetic DMARDs (csDMARDs).1,2 Here, we evaluated the efficacy and safety of UPA up to 5 yrs in a long-term extension (LTE) of SELECT-NEXT.
Methods: Patients received UPA 15 mg (UPA15) or 30 mg (UPA30) once daily or placebo (PBO) for 12 wks, each with stable background csDMARDs. Patients randomized to PBO were switched to UPA15/30 in a pre-specified manner at wk 12. From wk 12 onwards, patients were able to enter a blinded LTE for up to a total of 5 yrs, in which all patients received UPA15 or UPA30, and both the study site and patients were blinded to the UPA dose administered. The blinded extension remained until dose switching from UPA30 to UPA15, per protocol amendment, with the earliest switch occurring at wk 168. For patients not meeting CDAI ≤10 at or after wk 24, investigators were instructed to adjust background RA therapies. Efficacy data up to wk 260 are reported as observed (AO) for patients randomized to UPA15 or UPA30. Missing data for categorical endpoints were also imputed using NRI. Treatment-emergent adverse events (TEAEs) per 100 patient yrs (PY) were summarized over 5 yrs.
Results: Of the 661 patients randomized at baseline, 611 (92%) completed wk 12 and entered the 248-wk LTE. In total, 271 (41%) patients discontinued study drug during the LTE due to adverse events (16%), withdrawal of consent (10%), loss to follow-up (3%), lack of efficacy (3%), or other reasons (9%). Clinical outcomes continued to improve or were maintained through wk 260 (Figure 1), as demonstrated by 51% and 43% of patients achieving CDAI remission and 75% and 66% attaining DAS28(CRP) < 2.6 with UPA15 and UPA30, respectively (AO). ACR20/50/70 responses at wk 260 are shown in Figure 2, with over half of patients achieving ACR70 on either UPA dose (AO). Lower efficacy estimates based on NRI are also reported and were consistent for both dosage groups. Physical function and pain improved similarly with UPA15/30 at wk 260; the mean change from baseline was -0.86/-0.78 for HAQ-DI and -44/-44 mm for pain (AO). Patients who switched from PBO to UPA at wk 12 showed similar efficacy as those who were initiated on UPA (data not shown). Through 5 yrs, TEAEs and AEs of special interest were consistent with previous study-specific analyses (Table).2 Numerically higher rates of any AE, serious AEs, any infection, serious infections, herpes zoster, NMSC, and neutropenia were observed with UPA30 vs UPA15. While malignancies (excluding NMSC) and MACE were rare, numerically higher rates were also observed with UPA30 compared to UPA15.
Conclusion: UPA 15 mg or 30 mg therapy with background csDMARDs demonstrated sustained efficacy across multiple RA disease activity measures through the 5-yr study period. The safety profile was consistent with earlier time points and with an integrated phase 3 safety analysis of UPA in RA,3 although dose-dependent increases in the rates of certain adverse events were noted in patients receiving UPA30 vs UPA15.
1. Burmester, et al. Lancet 2018;391:2503–12
2. Burmester, et al. Ann Rheum Dis 2019;78:735–6
3. Cohen, et al. Ann Rheum Dis 2021: 80:304–11



Disclosures: G. Burmester, AbbVie, Galapagos, Lilly, MSD, Pfizer, Roche, UCB, Janssen, Gilead Sciences, Inc.; F. Van den bosch, AbbVie, Lilly, Galapagos, Janssen, Merck, Novartis, Pfizer, UCB, Amgen, Bristol-Myers Squibb(BMS), Celgene; J. Tesser, AbbVie, Amgen, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb (BMS), Eli Lilly, GlaxoSmithKlein (GSK), Janssen, Gilead, Merck/MSD, Novartis, Pfizer, UCB, Aurinia, Genentech, Sanofi-Genzyme, Biogen, Celgene, CSL Behring, Horizon, R-Pharma, Regeneron, Roche, Sandoz, Scipher, Takeda, Sun Pharma, Selecta, Vorso; A. Shmagel, AbbVie; Y. Duan, AbbVie; N. Khan, AbbVie; H. Camp, AbbVie; A. Kivitz, Amgen, Boehringer-Ingelheim, Janssen, Gilead, GlaxoSmithKlein (GSK), Novartis, Pfizer, Sanofi, Flexion, Eli Lilly, Genentech, UCB, AbbVie, Merck, ECOR1 CAPITAL, LLC, Chemocentryx, Regenerson, Grunenthal, Bendcare, Horizon.

