Back
Poster Session D
Epidemiology, health policy and outcomes
Session: (1750–1786) Epidemiology and Public Health Poster III
1756: Adverse Events in Patients with Inflammatory Joint Diseases: Results from the EULAR Coronavirus Vaccine (COVAX) Physician-reported Registry
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- AM
Ana Lúcia Martins Fernandes, MD
Centro Hospitalar Universitário do Algarve
Faro, Portugal
Abstract Poster Presenter(s)
Ana Lúcia Martins Fernandes1, Jose A Gomez-Puerta2, Juan Camilo Sarmiento-Monroy2, Saskia Lawson-Tovey3, Kimme Hyrich4, Laure Gossec5, Loreto Carmona6, Anja Strangfeld7, Elsa Mateus8, Ana Maria Rodrigues9, Eric Hachulla10, Marta Mosca11, Patrick Durez12, Bernd Raffeiner13, Nicolas Roux14, Viellard Eric15, Olivier Brocq16, Julija Zepa17, Inita Bulina18, Eva Strakova19, Vanda Mlynarikova20, Emoke Šteňová21, Martin Soubrier22, Xavier Mariette23 and Pedro Machado24, 1Centro Hospitalar Universitário do Algarve, Faro, Portugal, 2Hospital Clínic de Barcelona, Barcelona, Spain, 3Centre for Genetics and Genomics Versus Arthritis, Centre for Musculoskeletal Research, the University of Manchester, Manchester, UK AND National Institute of Health Research Manchester Biomedical Research Centre, Manchester University NHS Foundation Trust, Manchester Academic Health Science Centre, Manchester, United Kingdom, 4The University of Manchester, Manchester, United Kingdom, 5Sorbonne Université, Paris, France, 6Instituto de Salud Musculoesquelética (InMusc), Madrid, Spain, 7Deutsches Rheuma-Forschungszentrum Berlin, Berlin, Germany, 8EULAR, Lisboa, Portugal, 9Reuma.pt, Sociedade Portuguesa de Reumatologia, Lisbon, Portugal, 10University of Lille, LILLE, France, 11Rheumatology Unit, Department of Clinical and Experimental Medicine, University of Pisa, Pisa, Italy, 12Rheumatology, Cliniques Universitaires Saint-Luc – Université catholique de Louvain (UCLouvain) – Institut de Recherche Expérimentale et Clinique (IREC), Brussels, Belgium, 13Department of Rheumatology, Central Hospital of Bolzano, Bolzano, Italy, 14Service de Rhumatologie, Hôpital Robert Schuman, Metz, France, 15Private practice, St. Malo, France, 16Rheumatology- CH Princesse Grace, Monaco, Monaco, 17Riga Stradins University, Latvia, Pauls Stradins Clinical University Hospital, Centre of Rheumatology, Riga, Latvia, Riga, Latvia, 18Center of Rheumatology, Paul Stradins Clinical University hospital, Riga, Latvia, Riga, Latvia, 19Department of Internal Medicine, Faculty Hospital Prešov, Presov, Slovakia, 20National Institute of Rheumatic Diseases, Piešťany, Slovakia, 21University Hospital, Bratislava, Slovakia, 22Gabriel-Montpied Hospital, Clermont-Ferrand, France, 23Paris-Saclay University, Rueil Malmaison, Ile-de-France, France, 24University College London, London, United Kingdom
Background/Purpose: Patients with inflammatory/autoimmune rheumatic and musculoskeletal diseases (I-RMDs) were excluded from SARS-CoV-2 vaccination development programs. Therefore, concerns regarding the safety and effectiveness of SARS-CoV-2 vaccines in this population arose. Previous reports capturing a wide range of I-RMDs have been reassuring [1], but more granular data on specific conditions is desirable.
We aim to describe adverse events (AEs) in the most common inflammatory joint diseases (IJD), namely rheumatoid arthritis (RA), axial spondyloarthritis (axSpA), psoriatic arthritis (PsA), other peripheral spondyloarthritis (pSpA), and gout/other crystal arthritis (CA), in comparison with a group of patients with non-inflammatory rheumatic and musculoskeletal diseases (NI-RMDs).
Methods: Physician-reported registry of RMDs patients vaccinated against SARS-CoV-2. From 5 February 2021 to 3 March 2022, data were collected on demographics, vaccination, RMD diagnosis, immunomodulatory/immunosuppressive treatments and both early AEs and AEs of special interest. Data were analyzed descriptively.
Results: A total of 7625 patients from 31 different countries were included: 6870 with IJD (63.9% female, mean age 58.8 years), namely 3639 with RA, 1680 with axSpA, 1205 with PsA, 220 with pSpA and 126 with CA, and 755 with NI-RMDs (83.2% female, mean age 68.5 years). Main results are presented on Table 1. Most patients received a full scheme of vaccination (IJD: n=5964, 86.8%; NI-RMDs: n=612, 81.1%), and the most commonly administered vaccine was Pfizer/BioNTech (first dose: IJD n=4385, 63.8%; NI-RMDs n=534, 70.7%). AEs were observed less frequently in IJD than in NI-RMDs, including early AEs (vaccine reaction) (IJDs: n=3743, 54.5%; NI-RMDs: n=543, 71.9%) and AEs of special interest (IJDs: n=129, 1.9%; NI-RMDs: n=57, 7.5%). The pSpA group was an exception, presenting a higher rate of early AEs (n=185, 84.1%) and AEs of special interest (n=13, 5.9%). The overall rate of serious AEs was very low (IJD: n=22, 0.3%; NI-RMDs: n=19, 2.5%), and similar across IJDs. The serious AE included events of arrythmia, coronary heart disease, syncopes, arterial hypertension, telogen effluvium, eczema/rash, erythema nodosum, gengivitis, abdominal pain, lymphadenopathy, dyspnoea, pharyngitis exacerbation of asthma, thoracic pain, pulmonary embolism, herpes zoster and shingles. The registry being mainly dedicated to inflammatory RMDs, there was probably a bias favoring registration pf patients with mechanical RMDs having had AE. No deaths were reported and most patients recovered from the AE without sequelae.
Conclusion: Serious AEs were infrequently reported in patients with RA, PsA, axSpA, pSpA and CA. The safety profile of SARS-CoV-2 vaccines in patients with IJDs is reassuring.
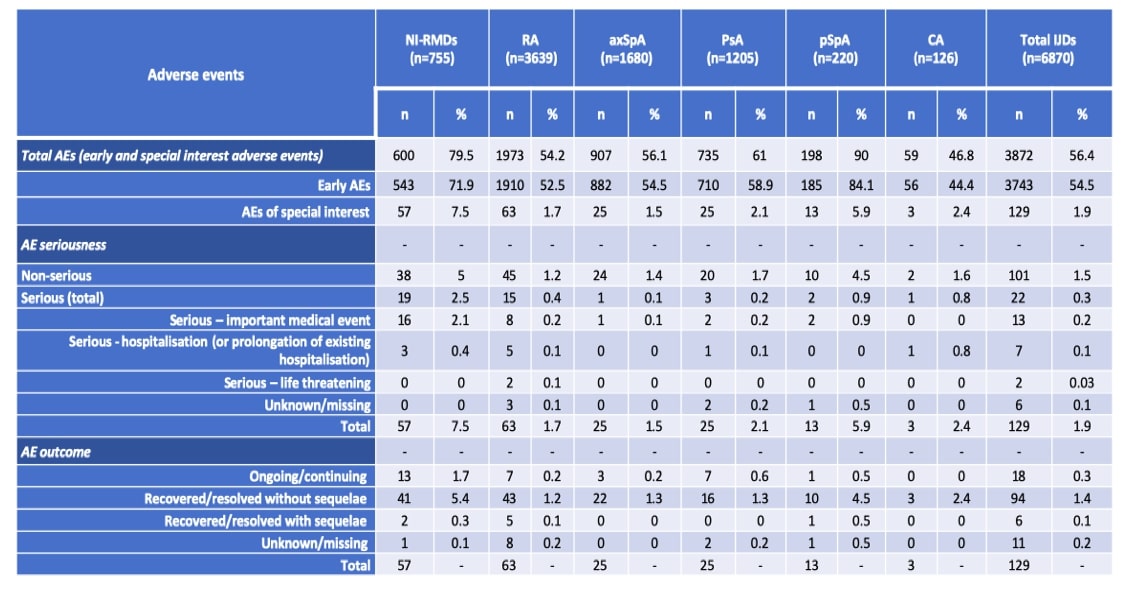 Table 1 Adverse events in patients with most common inflammatory joint diseases and non-inflammatory rheumatic and musculoskeletal diseases
Table 1 Adverse events in patients with most common inflammatory joint diseases and non-inflammatory rheumatic and musculoskeletal diseases
Disclosures: A. Martins Fernandes, None; J. Gomez-Puerta, GSK, Galapagos, Pfizer, Janssen, Sanofi, AbbVie, Bristol Myers Squibb, Lilly, Novartis, MSD, Roche; J. Sarmiento-Monroy, None; S. Lawson-Tovey, None; K. Hyrich, AbbVie/Abbott, Pfizer, Bristol-Myers Squibb(BMS); L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; L. Carmona, None; A. Strangfeld, AbbVie/Abbott, Merck/MSD, Roche, Bristol-Myers Squibb(BMS), Pfizer; E. Mateus, None; A. Rodrigues, None; E. Hachulla, GlaxoSmithKline, Johnson & Johnson, Roche-Chugai, CSL Behring, Bayer, Boehringer Ingelheim, Sanofi-Genzyme; M. Mosca, None; P. Durez, AbbVie, Galapagos, Lilly; B. Raffeiner, None; N. Roux, None; V. Eric, None; O. Brocq, None; J. Zepa, AbbVie/Abbott, Novartis, Janssen, AstraZeneca; I. Bulina, AbbVie/Abbott, AstraZeneca, Janssen, Novartis; E. Strakova, None; V. Mlynarikova, None; E. Šteňová, None; M. Soubrier, None; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos.
Background/Purpose: Patients with inflammatory/autoimmune rheumatic and musculoskeletal diseases (I-RMDs) were excluded from SARS-CoV-2 vaccination development programs. Therefore, concerns regarding the safety and effectiveness of SARS-CoV-2 vaccines in this population arose. Previous reports capturing a wide range of I-RMDs have been reassuring [1], but more granular data on specific conditions is desirable.
We aim to describe adverse events (AEs) in the most common inflammatory joint diseases (IJD), namely rheumatoid arthritis (RA), axial spondyloarthritis (axSpA), psoriatic arthritis (PsA), other peripheral spondyloarthritis (pSpA), and gout/other crystal arthritis (CA), in comparison with a group of patients with non-inflammatory rheumatic and musculoskeletal diseases (NI-RMDs).
Methods: Physician-reported registry of RMDs patients vaccinated against SARS-CoV-2. From 5 February 2021 to 3 March 2022, data were collected on demographics, vaccination, RMD diagnosis, immunomodulatory/immunosuppressive treatments and both early AEs and AEs of special interest. Data were analyzed descriptively.
Results: A total of 7625 patients from 31 different countries were included: 6870 with IJD (63.9% female, mean age 58.8 years), namely 3639 with RA, 1680 with axSpA, 1205 with PsA, 220 with pSpA and 126 with CA, and 755 with NI-RMDs (83.2% female, mean age 68.5 years). Main results are presented on Table 1. Most patients received a full scheme of vaccination (IJD: n=5964, 86.8%; NI-RMDs: n=612, 81.1%), and the most commonly administered vaccine was Pfizer/BioNTech (first dose: IJD n=4385, 63.8%; NI-RMDs n=534, 70.7%). AEs were observed less frequently in IJD than in NI-RMDs, including early AEs (vaccine reaction) (IJDs: n=3743, 54.5%; NI-RMDs: n=543, 71.9%) and AEs of special interest (IJDs: n=129, 1.9%; NI-RMDs: n=57, 7.5%). The pSpA group was an exception, presenting a higher rate of early AEs (n=185, 84.1%) and AEs of special interest (n=13, 5.9%). The overall rate of serious AEs was very low (IJD: n=22, 0.3%; NI-RMDs: n=19, 2.5%), and similar across IJDs. The serious AE included events of arrythmia, coronary heart disease, syncopes, arterial hypertension, telogen effluvium, eczema/rash, erythema nodosum, gengivitis, abdominal pain, lymphadenopathy, dyspnoea, pharyngitis exacerbation of asthma, thoracic pain, pulmonary embolism, herpes zoster and shingles. The registry being mainly dedicated to inflammatory RMDs, there was probably a bias favoring registration pf patients with mechanical RMDs having had AE. No deaths were reported and most patients recovered from the AE without sequelae.
Conclusion: Serious AEs were infrequently reported in patients with RA, PsA, axSpA, pSpA and CA. The safety profile of SARS-CoV-2 vaccines in patients with IJDs is reassuring.
 Table 1 Adverse events in patients with most common inflammatory joint diseases and non-inflammatory rheumatic and musculoskeletal diseases
Table 1 Adverse events in patients with most common inflammatory joint diseases and non-inflammatory rheumatic and musculoskeletal diseasesDisclosures: A. Martins Fernandes, None; J. Gomez-Puerta, GSK, Galapagos, Pfizer, Janssen, Sanofi, AbbVie, Bristol Myers Squibb, Lilly, Novartis, MSD, Roche; J. Sarmiento-Monroy, None; S. Lawson-Tovey, None; K. Hyrich, AbbVie/Abbott, Pfizer, Bristol-Myers Squibb(BMS); L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD; L. Carmona, None; A. Strangfeld, AbbVie/Abbott, Merck/MSD, Roche, Bristol-Myers Squibb(BMS), Pfizer; E. Mateus, None; A. Rodrigues, None; E. Hachulla, GlaxoSmithKline, Johnson & Johnson, Roche-Chugai, CSL Behring, Bayer, Boehringer Ingelheim, Sanofi-Genzyme; M. Mosca, None; P. Durez, AbbVie, Galapagos, Lilly; B. Raffeiner, None; N. Roux, None; V. Eric, None; O. Brocq, None; J. Zepa, AbbVie/Abbott, Novartis, Janssen, AstraZeneca; I. Bulina, AbbVie/Abbott, AstraZeneca, Janssen, Novartis; E. Strakova, None; V. Mlynarikova, None; E. Šteňová, None; M. Soubrier, None; X. Mariette, AstraZeneca, Bristol Myers Squibb, Galapagos, GSK, Novartis, Pfizer; P. Machado, AbbVie/Abbott, Eli Lilly, UCB, Novartis, Orphazyme, Galapagos.

