Back
Poster Session A
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (0403–0431) Spondyloarthritis Including PsA – Treatment Poster I: AxSpA
0425: Upadacitinib Pharmacokinetics and Exposure-Response Relationships for Efficacy and Safety in Axial Spondyloarthritis – Analyses of the Phase 3 Studies (SELECT-AXIS 1 and 2)
Saturday, November 12, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- MM
Mohamed-Eslam Mohamed, PhD
AbbVie
North Chicago, IL, United States
Abstract Poster Presenter(s)
Sumit Bhatnagar1, Doerthe Eckert2, Sven Stodtmann2, In-Ho Song3, Peter Wung3, Wei Liu3 and Mohamed-Eslam Mohamed3, 1AbbVie, Highland Park, IL, 2AbbVie, Ludwigshafen, Germany, 3AbbVie, Inc., North Chicago, IL
Background/Purpose: The efficacy and safety profile of 15 mg once-daily (QD) upadacitinib (UPA), an oral, reversible, JAK inhibitor has been established in axial spondyloarthritis (axSpA), which includes ankylosing spondylitis (AS) and non-radiographic axial spondyloarthritis (nr-axSpA), through the Phase 2/3 SELECT-AXIS 11 (AXIS 1) study and the Phase 3 studies within SELECT-AXIS 2 (AXIS 2). AXIS 1 studied AS subjects who were naïve to biologic disease modifying anti-rheumatic drug (bDMARD) and AXIS 2 included two independent studies: subjects with AS, who were bDMARD inadequate responders (IR), and subjects with nr-axSpA. The analyses presented here characterize UPA pharmacokinetics and exposure-response relationships for efficacy and safety endpoints using data from all three studies.
Methods: Pharmacokinetic samples were obtained from a pre-specified subset of bDMARD-IR AS (N = 81) and nr-axSpA (N = 71) subjects from AXIS 2 who were included in the analyses, while 92 subjects from AXIS 1 were included in the analyses. UPA pharmacokinetics were characterized in axSpA subjects using a previously established model based on data from healthy subjects and subjects with rheumatoid arthritis (RA)3. Covariates included in the model were indication (RA or axSpA compared to healthy subjects), creatinine clearance, and body weight on apparent oral clearance as well as body weight on apparent central volume of distribution. Efficacy data from the two studies with AS subjects were integrated for those who received placebo and UPA 15mg QD. UPA pharmacokinetics from AXIS 1 were previously analyzed2. Exposure-response analyses for efficacy were conducted using logistic regression modeling to characterize the relationships between UPA average plasma concentration during a dosing interval (Cavg) and the percentage of subjects achieving Assessment of SpondyloArthritis international Society (ASAS)20 and ASAS40 at Week 14. Exposure-response analyses for safety were conducted by graphical assessment for trends with UPA Cavg.
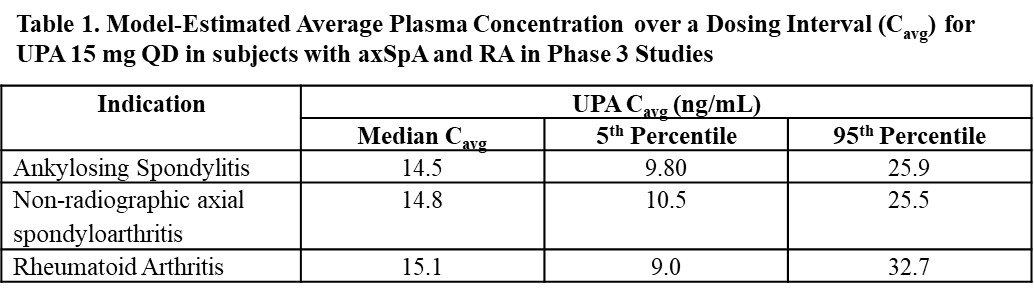
Results: The population pharmacokinetic model adequately described UPA pharmacokinetics in subjects with axSpA. UPA pharmacokinetics and model-predicted plasma exposures in subjects with axSpA were comparable to those in subjects with RA (Table 1).
Exposure-response analyses for efficacy demonstrated no clear exposure-response relationships for ASAS20 or ASAS40 in subjects with AS or nr-axSpA, indicating that exposures associated with UPA 15 mg QD maximized efficacy in subjects with axSpA. There were no clear trends for the relationships between UPA Cavg and the percentage of subjects experiencing lymphopenia (Grade ≥ 3), neutropenia (Grade ≥ 3), > 2 g/dL decrease in hemoglobin from baseline, any infection, serious infections, herpes zoster, or pneumonia through Week 14.
Conclusion: Pharmacokinetic analyses showed that UPA pharmacokinetics are similar between subjects with RA and axSpA. The exposure-response analyses demonstrated that plasma exposures associated with UPA 15 mg QD maximize efficacy in subjects with axSpA, supporting the use of UPA 15 mg QD as the optimal dose across the different axSpA disease subpopulations.

Disclosures: S. Bhatnagar, AbbVie; D. Eckert, AbbVie; S. Stodtmann, AbbVie; I. Song, AbbVie; P. Wung, AbbVie; W. Liu, AbbVie; M. Mohamed, Abbvie.
Background/Purpose: The efficacy and safety profile of 15 mg once-daily (QD) upadacitinib (UPA), an oral, reversible, JAK inhibitor has been established in axial spondyloarthritis (axSpA), which includes ankylosing spondylitis (AS) and non-radiographic axial spondyloarthritis (nr-axSpA), through the Phase 2/3 SELECT-AXIS 11 (AXIS 1) study and the Phase 3 studies within SELECT-AXIS 2 (AXIS 2). AXIS 1 studied AS subjects who were naïve to biologic disease modifying anti-rheumatic drug (bDMARD) and AXIS 2 included two independent studies: subjects with AS, who were bDMARD inadequate responders (IR), and subjects with nr-axSpA. The analyses presented here characterize UPA pharmacokinetics and exposure-response relationships for efficacy and safety endpoints using data from all three studies.
Methods: Pharmacokinetic samples were obtained from a pre-specified subset of bDMARD-IR AS (N = 81) and nr-axSpA (N = 71) subjects from AXIS 2 who were included in the analyses, while 92 subjects from AXIS 1 were included in the analyses. UPA pharmacokinetics were characterized in axSpA subjects using a previously established model based on data from healthy subjects and subjects with rheumatoid arthritis (RA)3. Covariates included in the model were indication (RA or axSpA compared to healthy subjects), creatinine clearance, and body weight on apparent oral clearance as well as body weight on apparent central volume of distribution. Efficacy data from the two studies with AS subjects were integrated for those who received placebo and UPA 15mg QD. UPA pharmacokinetics from AXIS 1 were previously analyzed2. Exposure-response analyses for efficacy were conducted using logistic regression modeling to characterize the relationships between UPA average plasma concentration during a dosing interval (Cavg) and the percentage of subjects achieving Assessment of SpondyloArthritis international Society (ASAS)20 and ASAS40 at Week 14. Exposure-response analyses for safety were conducted by graphical assessment for trends with UPA Cavg.
Results: The population pharmacokinetic model adequately described UPA pharmacokinetics in subjects with axSpA. UPA pharmacokinetics and model-predicted plasma exposures in subjects with axSpA were comparable to those in subjects with RA (Table 1).
Exposure-response analyses for efficacy demonstrated no clear exposure-response relationships for ASAS20 or ASAS40 in subjects with AS or nr-axSpA, indicating that exposures associated with UPA 15 mg QD maximized efficacy in subjects with axSpA. There were no clear trends for the relationships between UPA Cavg and the percentage of subjects experiencing lymphopenia (Grade ≥ 3), neutropenia (Grade ≥ 3), > 2 g/dL decrease in hemoglobin from baseline, any infection, serious infections, herpes zoster, or pneumonia through Week 14.
Conclusion: Pharmacokinetic analyses showed that UPA pharmacokinetics are similar between subjects with RA and axSpA. The exposure-response analyses demonstrated that plasma exposures associated with UPA 15 mg QD maximize efficacy in subjects with axSpA, supporting the use of UPA 15 mg QD as the optimal dose across the different axSpA disease subpopulations.


Disclosures: S. Bhatnagar, AbbVie; D. Eckert, AbbVie; S. Stodtmann, AbbVie; I. Song, AbbVie; P. Wung, AbbVie; W. Liu, AbbVie; M. Mohamed, Abbvie.

