Back
Poster Session D
Crystal arthropathies
Session: (1787–1829) Metabolic and Crystal Arthropathies – Basic and Clinical Science Poster
1821: Colchicine and Other Gout Medications and the Risk of COVID-19 Infection, Hospitalization, and Subsequent Outcomes in People with Gout
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
.png)
Jasvinder Singh, MD, MPH
University of Alabama at Birmingham
Birmingham, AL, United States
Abstract Poster Presenter(s)
Jasvinder singh1, Timothy Bergquist2, Vithal Madhira3 and Alfred Anzalone4, 1University of Alabama at Birmingham, Birmingham, AL, 2Sage Bionetworks, Seattle, WA, 3Palila Software, L.L.C., Reno, NV, 4University of Nebraska Medical Center, Ohama, NE
Background/Purpose: To examine whether the use of colchicine and other gout medications is associated with the risk of COVID-19 infection, hospitalization, and subsequent outcomes in patients with gout.
Methods: We used the US National COVID Cohort Collaborative (N3C), the largest US cohort of COVID-19 cases and demographically matched controls, to identify patients with gout (International Classification of Diseases (ICD)-10 code, M1A or M10). We used multivariable logistic regression to assess the association between colchicine use and the odds of COVID-19 and COVID-19 outcomes (hospitalization, intensive care unit (ICU) admission, 30-day mortality, and World Health Organization (WHO) classification for COVID-19 severity etc.), compared to non-use of each medication, adjusted for demographics, medical comorbidities, smoking status, body mass index, region, and COVID-19 treatments.
Results: The study cohort consisted of 147,060 people with gout; 54,727 (35%) had COVID-19 infection, 14,648 (26.8%) were hospitalized and 732 patients were exposed to colchicine within 30-days prior to their first COVID-19 diagnosis (1/1/2020 to 2/17/2022). Compared to non-use of the respective medication, colchicine use was associated with a decreased multivariable-adjusted odds ratio [aOR] (95% confidence interval [CI]) of COVID-19 infection, aOR 0.41 (95% CI, 0.35, 0.48) and allopurinol use was associated with an increased risk of COVID-19 infection, aOR 1.85 (95% CI, 1.58, 2.16); and anakinra use with higher 30-day mortality after COVID-19 infection, aOR 12.84 (1.76, 93.62). Colchicine use was not associated with significant differences in hospitalization, ICU admission, 30-day mortality, or COVID-19 severity (Figure). Results were confirmed in multiple sensitivity analyses.
Conclusion: Colchicine use in gout decreases and anakinra use increases the risk of COVID-19 infection. Patients with gout can consider the potential additional benefit/risk of continuing gout medications during the COVID-19 pandemic.
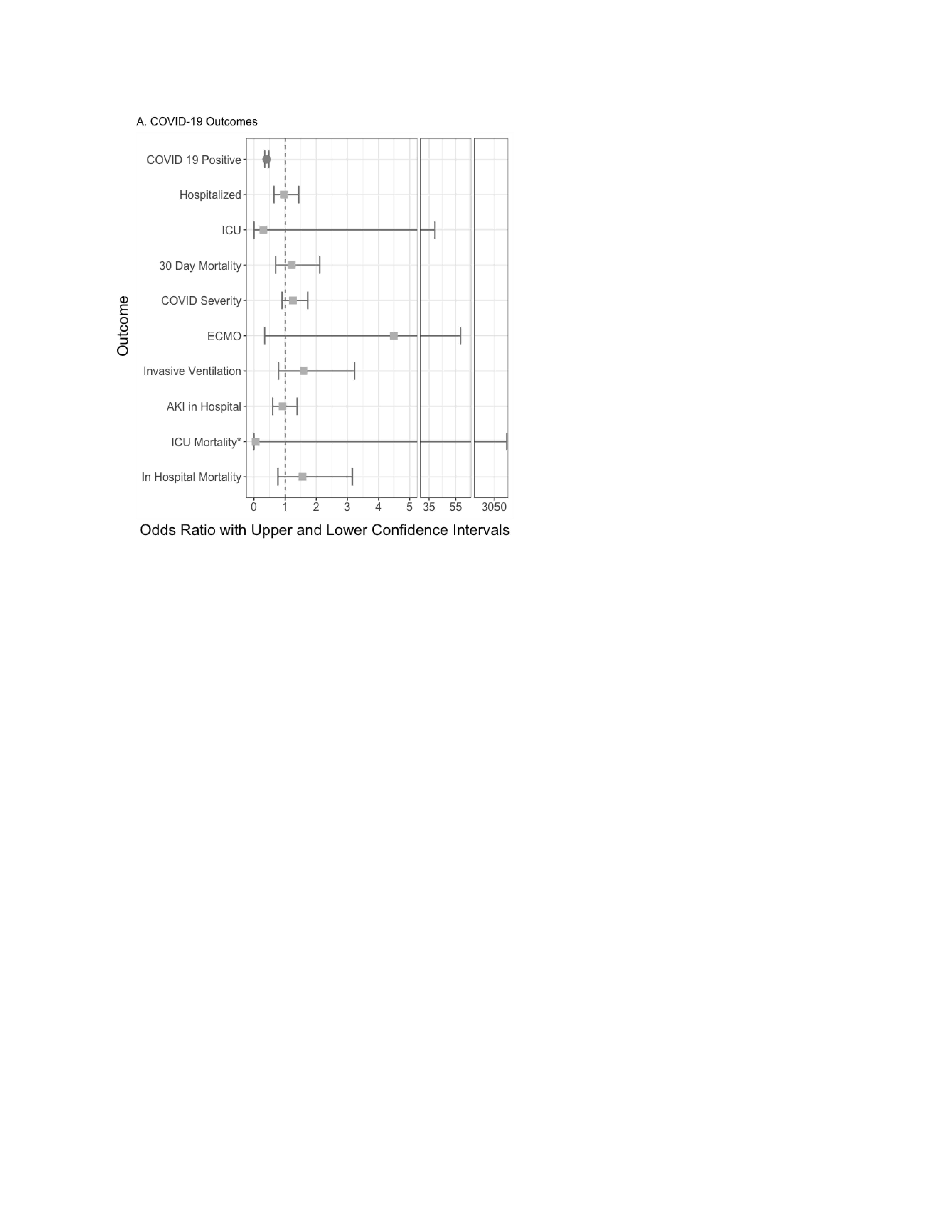 Figure 2. Multivariable-adjusted association of baseline colchicine use and each COVID-19 outcome with non-use as the referent category from US N3C cohort, March 1, 2020-October 14, 2021
Figure 2. Multivariable-adjusted association of baseline colchicine use and each COVID-19 outcome with non-use as the referent category from US N3C cohort, March 1, 2020-October 14, 2021
Figure 2 Legend: The figure shows the odds ratios and 95% confidence interval for the association between colchicine use and COVID-19-outcomes
AKI, acute kidney injury; CI: Confidence interval; ICU, intensive care unit; LOS: Length of stay
Hospitalized: adjusted for demographics, weight categories per BMI as normal vs. underweight, overweight, and obese, smoking status, US region, and modified Deyo-Charlson index
All other outcomes: adjusted for the above variables and all COVID-19 treatments
Circles (red) denote significant outcomes, orange squares denote non-significant outcomes.
*COVID outcomes did not have a sufficient sample size to calculate valid odds ratios.
Disclosures: J. singh, Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two Labs Inc., Adept Field Solutions, Clinical Care Options, Clearview Healthcare Partners, Putnam Associates, Focus Forward, Navigant Consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, Practice Point Communications, National Institutes of Health, American College of Rheumatology, Zimmer Biomet Holdings, Intuitive Surgical Inc./Philips Electronics North America, TPT Global Tech, Vaxart Pharmaceuticals, Atyu Biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix Pharmaceuticals Holding Corp, Charlotte's Web Holdings, Inc., Amarin, Viking, Moderna Pharmaceuticals, Simply Speaking, Outcomes Measures in Rheumatology (OMERACT), FDA Arthritis Advisory Committee, Veterans Affairs Rheumatology Field Advisory Board (FAB), University of Alabama at Birmingham (UAB) Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis; T. Bergquist, None; V. Madhira, None; A. Anzalone, None.
Background/Purpose: To examine whether the use of colchicine and other gout medications is associated with the risk of COVID-19 infection, hospitalization, and subsequent outcomes in patients with gout.
Methods: We used the US National COVID Cohort Collaborative (N3C), the largest US cohort of COVID-19 cases and demographically matched controls, to identify patients with gout (International Classification of Diseases (ICD)-10 code, M1A or M10). We used multivariable logistic regression to assess the association between colchicine use and the odds of COVID-19 and COVID-19 outcomes (hospitalization, intensive care unit (ICU) admission, 30-day mortality, and World Health Organization (WHO) classification for COVID-19 severity etc.), compared to non-use of each medication, adjusted for demographics, medical comorbidities, smoking status, body mass index, region, and COVID-19 treatments.
Results: The study cohort consisted of 147,060 people with gout; 54,727 (35%) had COVID-19 infection, 14,648 (26.8%) were hospitalized and 732 patients were exposed to colchicine within 30-days prior to their first COVID-19 diagnosis (1/1/2020 to 2/17/2022). Compared to non-use of the respective medication, colchicine use was associated with a decreased multivariable-adjusted odds ratio [aOR] (95% confidence interval [CI]) of COVID-19 infection, aOR 0.41 (95% CI, 0.35, 0.48) and allopurinol use was associated with an increased risk of COVID-19 infection, aOR 1.85 (95% CI, 1.58, 2.16); and anakinra use with higher 30-day mortality after COVID-19 infection, aOR 12.84 (1.76, 93.62). Colchicine use was not associated with significant differences in hospitalization, ICU admission, 30-day mortality, or COVID-19 severity (Figure). Results were confirmed in multiple sensitivity analyses.
Conclusion: Colchicine use in gout decreases and anakinra use increases the risk of COVID-19 infection. Patients with gout can consider the potential additional benefit/risk of continuing gout medications during the COVID-19 pandemic.
 Figure 2. Multivariable-adjusted association of baseline colchicine use and each COVID-19 outcome with non-use as the referent category from US N3C cohort, March 1, 2020-October 14, 2021
Figure 2. Multivariable-adjusted association of baseline colchicine use and each COVID-19 outcome with non-use as the referent category from US N3C cohort, March 1, 2020-October 14, 2021Figure 2 Legend: The figure shows the odds ratios and 95% confidence interval for the association between colchicine use and COVID-19-outcomes
AKI, acute kidney injury; CI: Confidence interval; ICU, intensive care unit; LOS: Length of stay
Hospitalized: adjusted for demographics, weight categories per BMI as normal vs. underweight, overweight, and obese, smoking status, US region, and modified Deyo-Charlson index
All other outcomes: adjusted for the above variables and all COVID-19 treatments
Circles (red) denote significant outcomes, orange squares denote non-significant outcomes.
*COVID outcomes did not have a sufficient sample size to calculate valid odds ratios.
Disclosures: J. singh, Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two Labs Inc., Adept Field Solutions, Clinical Care Options, Clearview Healthcare Partners, Putnam Associates, Focus Forward, Navigant Consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, Practice Point Communications, National Institutes of Health, American College of Rheumatology, Zimmer Biomet Holdings, Intuitive Surgical Inc./Philips Electronics North America, TPT Global Tech, Vaxart Pharmaceuticals, Atyu Biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix Pharmaceuticals Holding Corp, Charlotte's Web Holdings, Inc., Amarin, Viking, Moderna Pharmaceuticals, Simply Speaking, Outcomes Measures in Rheumatology (OMERACT), FDA Arthritis Advisory Committee, Veterans Affairs Rheumatology Field Advisory Board (FAB), University of Alabama at Birmingham (UAB) Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis; T. Bergquist, None; V. Madhira, None; A. Anzalone, None.

