Back
Poster Session D
Osteoarthritis (OA) and related disorders
Session: (1888–1923) Osteoarthritis – Clinical Poster
1900: CoLchicine for Treatment of OsteoArthritis of the Knee (CLOAK)-A Double-blind, Placebo-controlled Trial
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JS
Jonathan Samuels, MD, RhMSUS
NYU Langone
Rye Brook, NY, United States
Abstract Poster Presenter(s)
Jonathan Samuels1, Michael Pillinger2, Michael Toprover3, Svetlana Krasnokutsky Samuels4, Apoorva Patil5, Fernando Bomfim6, Renata La Rocca Vieira6, David Wei5, Sydney Catron5, Maryfe coronel5, Annie Kim5 and Sarah Moussavi5, 1NYU Langone, Rye Brook, NY, 2NYU Grossman School of Medicine, New York, NY, 3Division of Rheumatology, New York University Grossman School of Medicine and Rheumatology Section, New York Harbor Health Care System, New York Campus of the U.S. Department of Veterans Affairs, New York, NY, 4Westmed, Rye Brook, NY, 5NYU Langone Health, New York, NY, 6NYU Langone, New York, NY
Background/Purpose: Knee osteoarthritis (OA) is an inflammatory disease, with a probable role for IL-1b. Calcium and urate crystals may promote OA by activating the NLRP3 inflammasome to produce IL-1b. Colchicine is a well-tolerated anti-inflammatory agent that inhibits the inflammasome and suppresses IL-1b. Studies examining the impact of colchicine on knee OA have yielded varying results, with some reporting pain relief, others improvement of inflammatory markers, and none assessing synovial effusions. We report the interim, blinded results of our ongoing colchicine trial for knee OA.
Methods: CLOAK is a randomized, double-blind, placebo-controlled trial of colchicine (once daily for 3 months) (Figure 1). We are enrolling subjects ≥ 40 years of age, with symptomatic knee OA, Kellgren-Lawrence grade 2 or 3 radiographs, and willingness to forego other anti-inflammatory therapy during the trial. The primary outcome is the change in knee pain by visual analog scale (VAS) after 3 months of treatment, comparing the colchicine and placebo groups. Secondary outcomes include pre to post treatment Knee Injury and Osteoarthritis Outcome Score (KOOS), mean doses of analgesics used, and changes in plasma and peripheral blood leukocyte inflammatory markers. Patients undergo knee ultrasound (US) pre- and post-treatment to assess synovitis and effusion. We aspirate synovial fluid when appropriate, and will analyze all available blood and synovial samples after study completion.
Results: To date, 715 potential subjects have been contacted, 82 screened, and 71 enrolled. Among 60 who have completed the study, 51.6% are male, 60% White, 30% Black, 3.3% Asian and 6.7% other, with mean BMI of 27.6 kg/m2 and age of 66.8 years. The mean VAS pain score among all completing participants (subjects and controls combined) improved by 0.98 units in the index knee, and mean KOOS scores improved for symptoms, pain, activities of daily living (ADL), sports activity, and quality of life (QOL). Overall 36 (60%) demonstrated VAS improvement (mean improvement 2.3) whereas 24 (40%) demonstrated no change or worsening. Overall, subjects whose VAS improved showed concordant improvement in the KOOS: mean symptoms by 10.5, pain by 12.4, ADL by 14.8, sports activity by 5.8 and QOL by 11.4 units. The subsets of patients with baseline VAS ≥6 and baseline KOOS ≤60 (i.e., more severe) showed significantly more 3-month KOOS pain improvement, even with the blinded inclusion of placebo (Figure 2). All underwent US at baseline and 3 months. Among 36 patients with VAS improvement over 3 months, 6 had baseline synovial effusions ≥4 mm (in longitudinal and transverse views) and 5 of these effusions were smaller on US post-treatment and one remained stable.
Conclusion: The results of this blinded analysis are consistent with a potential benefit of colchicine for pain, function and effusion in subjects not taking other anti-inflammatory agents. Enrollment is ongoing and the study will be unblinded and fully analyzed after completion.
.jpg) Figure 1. Flow diagram of study plan.
Figure 1. Flow diagram of study plan.
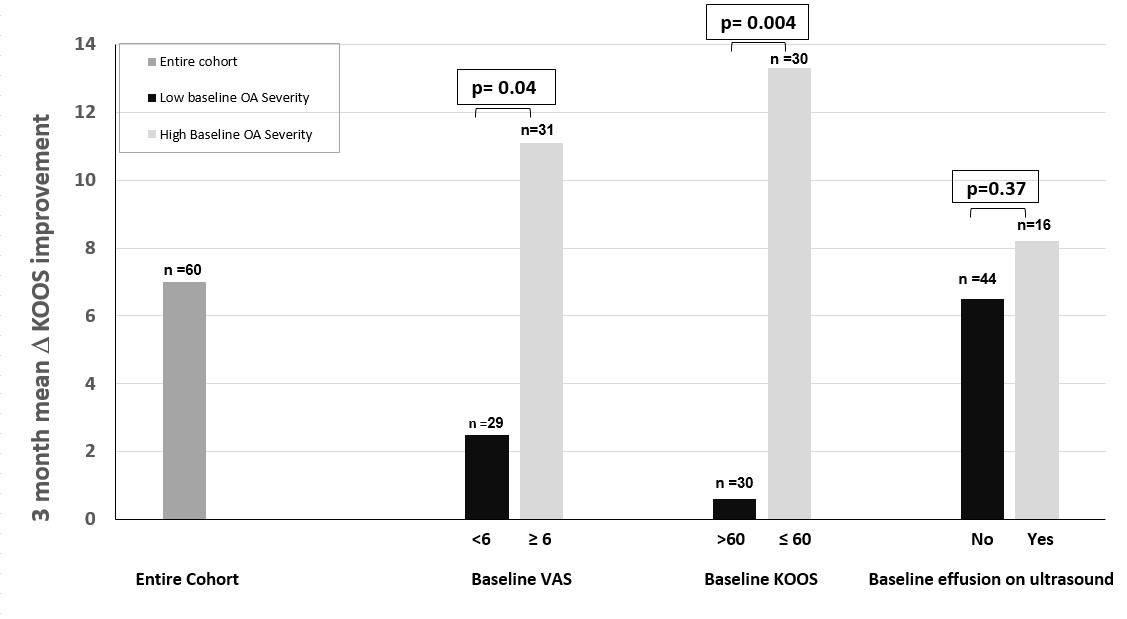 Figure 2. Subject improvement in KOOS score from beginning to end of study, according to high or low baseline severity as measured by VAS and KOOS scores and presence of synovial effusion.
Figure 2. Subject improvement in KOOS score from beginning to end of study, according to high or low baseline severity as measured by VAS and KOOS scores and presence of synovial effusion.
Disclosures: J. Samuels, None; M. Pillinger, Horizon Therapeutics, Sobi, Fortress Bioscience, Hikma; M. Toprover, Horizon Pharma; S. Krasnokutsky Samuels, None; A. Patil, None; F. Bomfim, None; R. La Rocca Vieira, None; D. Wei, None; S. Catron, None; M. coronel, None; A. Kim, None; S. Moussavi, None.
Background/Purpose: Knee osteoarthritis (OA) is an inflammatory disease, with a probable role for IL-1b. Calcium and urate crystals may promote OA by activating the NLRP3 inflammasome to produce IL-1b. Colchicine is a well-tolerated anti-inflammatory agent that inhibits the inflammasome and suppresses IL-1b. Studies examining the impact of colchicine on knee OA have yielded varying results, with some reporting pain relief, others improvement of inflammatory markers, and none assessing synovial effusions. We report the interim, blinded results of our ongoing colchicine trial for knee OA.
Methods: CLOAK is a randomized, double-blind, placebo-controlled trial of colchicine (once daily for 3 months) (Figure 1). We are enrolling subjects ≥ 40 years of age, with symptomatic knee OA, Kellgren-Lawrence grade 2 or 3 radiographs, and willingness to forego other anti-inflammatory therapy during the trial. The primary outcome is the change in knee pain by visual analog scale (VAS) after 3 months of treatment, comparing the colchicine and placebo groups. Secondary outcomes include pre to post treatment Knee Injury and Osteoarthritis Outcome Score (KOOS), mean doses of analgesics used, and changes in plasma and peripheral blood leukocyte inflammatory markers. Patients undergo knee ultrasound (US) pre- and post-treatment to assess synovitis and effusion. We aspirate synovial fluid when appropriate, and will analyze all available blood and synovial samples after study completion.
Results: To date, 715 potential subjects have been contacted, 82 screened, and 71 enrolled. Among 60 who have completed the study, 51.6% are male, 60% White, 30% Black, 3.3% Asian and 6.7% other, with mean BMI of 27.6 kg/m2 and age of 66.8 years. The mean VAS pain score among all completing participants (subjects and controls combined) improved by 0.98 units in the index knee, and mean KOOS scores improved for symptoms, pain, activities of daily living (ADL), sports activity, and quality of life (QOL). Overall 36 (60%) demonstrated VAS improvement (mean improvement 2.3) whereas 24 (40%) demonstrated no change or worsening. Overall, subjects whose VAS improved showed concordant improvement in the KOOS: mean symptoms by 10.5, pain by 12.4, ADL by 14.8, sports activity by 5.8 and QOL by 11.4 units. The subsets of patients with baseline VAS ≥6 and baseline KOOS ≤60 (i.e., more severe) showed significantly more 3-month KOOS pain improvement, even with the blinded inclusion of placebo (Figure 2). All underwent US at baseline and 3 months. Among 36 patients with VAS improvement over 3 months, 6 had baseline synovial effusions ≥4 mm (in longitudinal and transverse views) and 5 of these effusions were smaller on US post-treatment and one remained stable.
Conclusion: The results of this blinded analysis are consistent with a potential benefit of colchicine for pain, function and effusion in subjects not taking other anti-inflammatory agents. Enrollment is ongoing and the study will be unblinded and fully analyzed after completion.
.jpg) Figure 1. Flow diagram of study plan.
Figure 1. Flow diagram of study plan. Figure 2. Subject improvement in KOOS score from beginning to end of study, according to high or low baseline severity as measured by VAS and KOOS scores and presence of synovial effusion.
Figure 2. Subject improvement in KOOS score from beginning to end of study, according to high or low baseline severity as measured by VAS and KOOS scores and presence of synovial effusion.Disclosures: J. Samuels, None; M. Pillinger, Horizon Therapeutics, Sobi, Fortress Bioscience, Hikma; M. Toprover, Horizon Pharma; S. Krasnokutsky Samuels, None; A. Patil, None; F. Bomfim, None; R. La Rocca Vieira, None; D. Wei, None; S. Catron, None; M. coronel, None; A. Kim, None; S. Moussavi, None.

