Back
Abstract Session
Fibrosing rheumatic diseases (scleroderma, MCTD, IgG4-related disease, scleroderma mimics)
Session: Abstracts: Systemic Sclerosis and Related Disorders – Clinical I: Trials and Therapeutics (0518–0523)
0520: Combination Therapy of Mycophenolate Mofetil and Pirfenidone vs. Mycophenolate Alone: Results from the Scleroderma Lung Study III
Saturday, November 12, 2022
3:30 PM – 3:40 PM Eastern Time
Location: Room 121

Dinesh Khanna, MD, MSc
University of Michigan
Ann Arbor, MI, United States
Presenting Author(s)
Dinesh Khanna1, Cathie Spino2, Elana Bernstein3, Jonathan Goldin4, Donald Tashkin4, Michael roth4 and On Behalf of SLS III Investigators2, 1Division of Rheumatology, Department of Internal Medicine, Scleroderma Program, University of Michigan, Ann Arbor, MI, 2University of Michigan, Ann Arbor, MI, 3Columbia University, New York, NY, 4University of California Los Angeles, Los Angeles, CA
Background/Purpose: Scleroderma Lung Study (SLS) II established mycophenolate mofetil (MMF) as an active therapy for scleroderma-related interstitial lung disease (SSc-ILD) and the need to consider background MMF in trial designs. SLS III envisioned an upfront combination therapy in which the rapid onset and anti-fibrotic effects of pirfenidone (PFD) would complement the delayed immunosuppressive and anti-inflammatory effects of MMF.
Methods: An investigator-initiated multi-center, double-blind, placebo (PLA)-controlled Phase II trial evaluated combination therapy (MMF+PFD vs MMF+PLA) in those with SSc-ILD. Key inclusion criteria were age≥18, SSc-ILD with symptoms (Mahler dyspnea index), FVC% ≤ 85% predicted, disease duration ≤7 yrs, and any ground glass opacity on HRCT. Subjects were randomized to each arm, stratified by site and prior MMF therapy (naïve, >0 to ≤3 months, and >3 months to 6 months) with a planned enrollment of 150. The primary endpoint was the change from baseline in the mean forced vital capacity % predicted (FVC%) over the course of the 18-month treatment period. Treatment differences were tested using linear mixed effects models for longitudinal endpoints and ANCOVA for HRCT endpoints measured at screening and 18 months, both adjusting for prior MMF therapy and efficacy measure at screening or baseline.
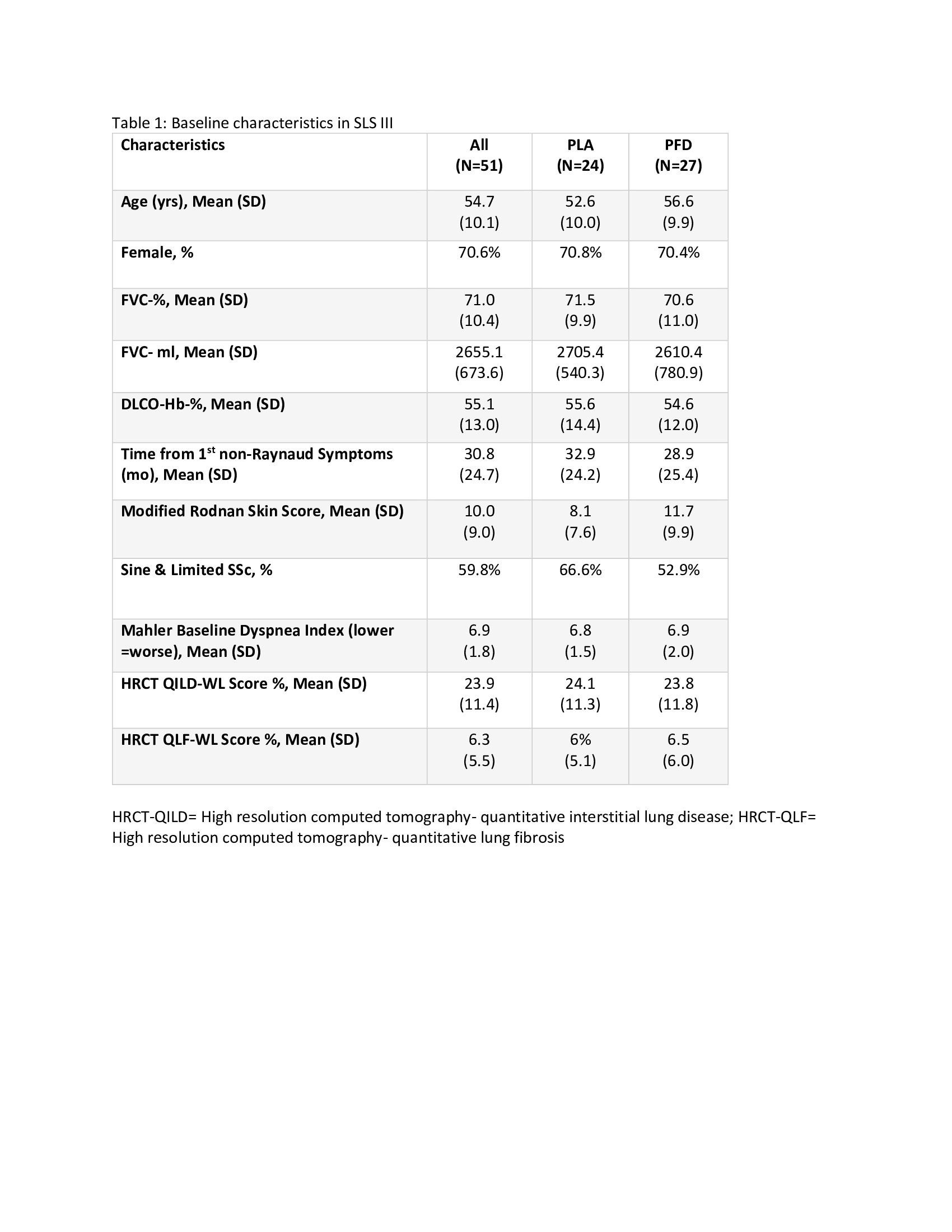
Results: 51 participants were randomized (76% naive; 14% ≤3 months MMF; 10% >3-6 months MMF) with 27 to MMF+PFD and 24 to MMF+PLA. Recruitment was prematurely stopped due to COVID-19 and the impact of prior drug treatment on eligibility. Table 1 details baseline characteristics. Overall, a similar magnitude of improvement in FVC% over 18 months occurred in both arms (2.24% MMF+PLA vs. 2.09% MMF+PFD; P=0.93) with a more rapid improvement noted in the MMF+PFD arm over 6 months (Figure 1). In addition, there were trends favoring the PFD arm in HRCT quantification of fibrosis (QLF) and interstitial lung disease (QILD) as well as in HAQ-DI, SGRQ-activity scale, and PROMIS-29 physical function scales, all meeting/exceeding minimally important differences (Table 2). 74.1% of subjects on MMF+PFD vs 29.2% on MMF+PLA had adverse events of special interest; the difference primarily related to gastrointestinal disorders (55.6% vs. 20.8%) and photosensitivity (14.8% vs 0%). 7 participants prematurely discontinued one or both study drugs, all in the MMF+PFD arm. There were no deaths.
Conclusion: Consistent with a primary hypothesis, upfront combination therapy (MMF+PFD) led to more rapid improvement in the FVC% (0-6 months) but with a similar overall improvement over 18 months as compared to MMF +PLA. Changes in HRCT outcomes and patient-reported outcomes tended to favor MMF+PFD. Side effects were similar to the published literature and more common with MMF+ PFD. Although the study was underpowered, it raises the question whether upfront MMF+PFD can lead to a more rapid improvement in FVC and greater impact on HRCT and patient-reported outcomes.
.jpg)
.jpg)
Disclosures: D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences; C. Spino, None; E. Bernstein, Boehringer-Ingelheim, Kadmon, Pfizer; J. Goldin, MedQIA, LLC; D. Tashkin, Genentech; M. roth, Genentech; O. SLS III Investigators, None.
Background/Purpose: Scleroderma Lung Study (SLS) II established mycophenolate mofetil (MMF) as an active therapy for scleroderma-related interstitial lung disease (SSc-ILD) and the need to consider background MMF in trial designs. SLS III envisioned an upfront combination therapy in which the rapid onset and anti-fibrotic effects of pirfenidone (PFD) would complement the delayed immunosuppressive and anti-inflammatory effects of MMF.
Methods: An investigator-initiated multi-center, double-blind, placebo (PLA)-controlled Phase II trial evaluated combination therapy (MMF+PFD vs MMF+PLA) in those with SSc-ILD. Key inclusion criteria were age≥18, SSc-ILD with symptoms (Mahler dyspnea index), FVC% ≤ 85% predicted, disease duration ≤7 yrs, and any ground glass opacity on HRCT. Subjects were randomized to each arm, stratified by site and prior MMF therapy (naïve, >0 to ≤3 months, and >3 months to 6 months) with a planned enrollment of 150. The primary endpoint was the change from baseline in the mean forced vital capacity % predicted (FVC%) over the course of the 18-month treatment period. Treatment differences were tested using linear mixed effects models for longitudinal endpoints and ANCOVA for HRCT endpoints measured at screening and 18 months, both adjusting for prior MMF therapy and efficacy measure at screening or baseline.
Results: 51 participants were randomized (76% naive; 14% ≤3 months MMF; 10% >3-6 months MMF) with 27 to MMF+PFD and 24 to MMF+PLA. Recruitment was prematurely stopped due to COVID-19 and the impact of prior drug treatment on eligibility. Table 1 details baseline characteristics. Overall, a similar magnitude of improvement in FVC% over 18 months occurred in both arms (2.24% MMF+PLA vs. 2.09% MMF+PFD; P=0.93) with a more rapid improvement noted in the MMF+PFD arm over 6 months (Figure 1). In addition, there were trends favoring the PFD arm in HRCT quantification of fibrosis (QLF) and interstitial lung disease (QILD) as well as in HAQ-DI, SGRQ-activity scale, and PROMIS-29 physical function scales, all meeting/exceeding minimally important differences (Table 2). 74.1% of subjects on MMF+PFD vs 29.2% on MMF+PLA had adverse events of special interest; the difference primarily related to gastrointestinal disorders (55.6% vs. 20.8%) and photosensitivity (14.8% vs 0%). 7 participants prematurely discontinued one or both study drugs, all in the MMF+PFD arm. There were no deaths.
Conclusion: Consistent with a primary hypothesis, upfront combination therapy (MMF+PFD) led to more rapid improvement in the FVC% (0-6 months) but with a similar overall improvement over 18 months as compared to MMF +PLA. Changes in HRCT outcomes and patient-reported outcomes tended to favor MMF+PFD. Side effects were similar to the published literature and more common with MMF+ PFD. Although the study was underpowered, it raises the question whether upfront MMF+PFD can lead to a more rapid improvement in FVC and greater impact on HRCT and patient-reported outcomes.

.jpg)
.jpg)
Disclosures: D. Khanna, Boehringer Ingelheim, Genentech, Prometheus, Horizon, Chemomab, Talaris, Gesynta, Amgen, Acceleron, Actelion, Bayer, CSL Behring, Paracrine Cell Therapy, Mitsubishi Tanabe, Theraly, Eicos Sciences; C. Spino, None; E. Bernstein, Boehringer-Ingelheim, Kadmon, Pfizer; J. Goldin, MedQIA, LLC; D. Tashkin, Genentech; M. roth, Genentech; O. SLS III Investigators, None.

