Back
Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, and Outcomes I: Renal Aspects (0536–0541)
0536: Change in Urinary Biomarkers at Three Months Predicts 1-year Treatment Response of Lupus Nephritis Better Than Proteinuria
Saturday, November 12, 2022
4:30 PM – 4:40 PM Eastern Time
Location: Room 122

Andrea Fava, MD
Johns Hopkins University
Baltimore, MD, United States
Presenting Author(s)
Andrea Fava1, Laurence S Magder2, Daniel W. Goldman3, Jill Buyon4, Betty Diamond5, Joel Guthridge6, William Apruzzese7, Derek Fine1, Jose Monroy-Trujillo1, Mohamed G. Atta1, Peter Izmirly4, H Michael Belmont8, Anne Davidson5, Maria Dall'Era9, Deepak Rao7, Arnon Arazi10, Nir Hacohen11, Soumya Raychaudhuri7, the Accelerating Medicines Partnership (AMP) RA/SLE12 and Michelle Petri3, 1Johns Hopkins University, Baltimore, MD, 2University of Maryland, Department of Epidemiology and Public Health, Baltimore, MD, 3Johns Hopkins University School of Medicine, Division of Rheumatology, Baltimore, MD, 4NYU Grossman School of Medicine, New York, NY, 5Feinstein Institutes for Medical Research, Manhasset, NY, 6Oklahoma Medical Research Foundation, Oklahoma City, OK, 7Brigham and Women's Hospital, Boston, MA, 8NYU School of Medicine, New York, NY, 9University of California, San Francisco, San Francisco, 10Feinstein Institutes for Medical Research, Melrose, MA, 11Broad Institute, Cambridge, MA, 12Multiple Insitutions
Background/Purpose: A decline of urine protein-to-creatinine ratio (UPCR) to < 0.5 is associated with better long-term preservation of kidney function in lupus nephritis (LN). UPCR < 0.5 defines complete response in guidelines and clinical trials when achieved after 1 or 2 years. Biomarkers of early response are needed to guide early treatment changes. We studied longitudinal urine proteomic profiles in LN to identify early predictors of proteinuric response.
Methods: We quantified 1200 biomarkers (Kiloplex, RayBiotech) in urine samples collected on the day of (73%) or within 3 weeks (27%) of kidney biopsy and week 12, 24, or 52 in LN patients (ISN class III, IV, V, or mixed) with proteinuria > 1 g/d. Response was defined at one year from renal biopsy: Complete = UPCR < 0.5, serum creatinine (sCr) < 125% of baseline, prednisone ≤ 10mg/d; Partial = UPCR < 50% from baseline but >0.5, sCr < 125% of baseline, but prednisone allowed to 15 mg/d; Non responder = not meeting previous definitions.
Results: A total of 127 patients were included: 48 (38%) with pure proliferative LN (class III or IV), 41 (32%) with mixed LN (III or IV +/- V), and 38% (30%) with pure membranous LN. Response was complete in 34 (27%), partial in 29 (23%), and none in 64 (50%). There were no urinary biomarkers at baseline that predicted response. We then analyzed the changes in urinary proteins at 3 months compared to baseline. Patients who responded at 1 year showed an early decline in 51 urinary proteins led by CD163, IL-16, and macrophage mannose receptor (MMR, CD206) (Figure 1A) which matched the proteomic signature associated with histological activity (Figure 1B). No changes were observed in nonresponders. The decline of several urinary biomarkers at 3 months outperformed a decline in UPCR (clinical standard) in predicting the 1 year response (Figure 2A). In particular, a decline of CD163 predicted 1 year response in ROC analysis with an area under the curve (AUC) of 83% compared to an AUC of 75% for UPCR decline (Figure 2B). In proliferative LN, urinary biomarkers displayed superior performance with an AUC of 91%, 86%, and 78% for the decline of MMR, CD163, and UPCR, respectively (Figure 2C-D). Pathway enrichment analysis identified leukocyte activation, neutrophil degranulation, and matrix degradation as the main pathways reduced at 3 months in responders.
Conclusion: An early decline in urinary biomarkers of histological activity is associated with proteinuric response at 1 year. These findings indicate that effective immunosuppression induces by three months an immunological response in the kidney that can be noninvasively monitored in the urine. Biomarkers of immunological response outperformed early decline of UPCR, the standard of care, in predicting 1-year proteinuric response, especially in proliferative LN. Because biomarkers of immunological response parallel intrarenal activity, they could detect early treatment response/failure and allow early treatment changes. They could serve as surrogate endpoints in clinical trials. Longitudinal studies are needed to confirm that this immunological response is a better predictor of long-term kidney function preservation than proteinuric responses.
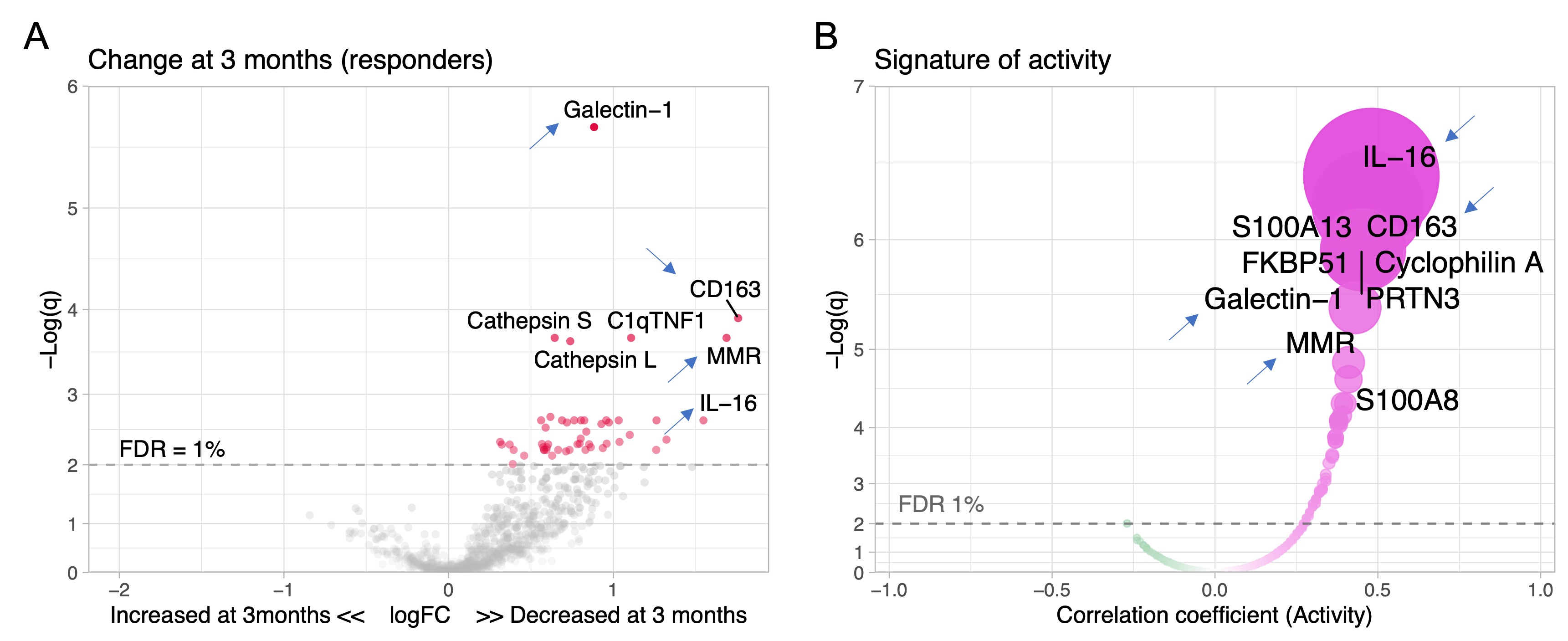 Figure 1. Proteins linked to intrarenal activity (NIH activity index) decrease in treatment responders. Volcano plot of the changes of the urinary proteomic profile of treatment responders at 3 months (A) after kidney biopsy/treatment compared to baseline at time of biopsy. (B) Volcano plot displaying the Spearman’s correlation coefficients of urinary biomarkers at time of biopsy with histological activity defined as the NIH activity index. Blue arrows indicate 4 examples of shared urinary biomarkers that indicate both intrarenal activity and response to treatment. FDR, false discovery rate; q, adjusted p value.
Figure 1. Proteins linked to intrarenal activity (NIH activity index) decrease in treatment responders. Volcano plot of the changes of the urinary proteomic profile of treatment responders at 3 months (A) after kidney biopsy/treatment compared to baseline at time of biopsy. (B) Volcano plot displaying the Spearman’s correlation coefficients of urinary biomarkers at time of biopsy with histological activity defined as the NIH activity index. Blue arrows indicate 4 examples of shared urinary biomarkers that indicate both intrarenal activity and response to treatment. FDR, false discovery rate; q, adjusted p value.
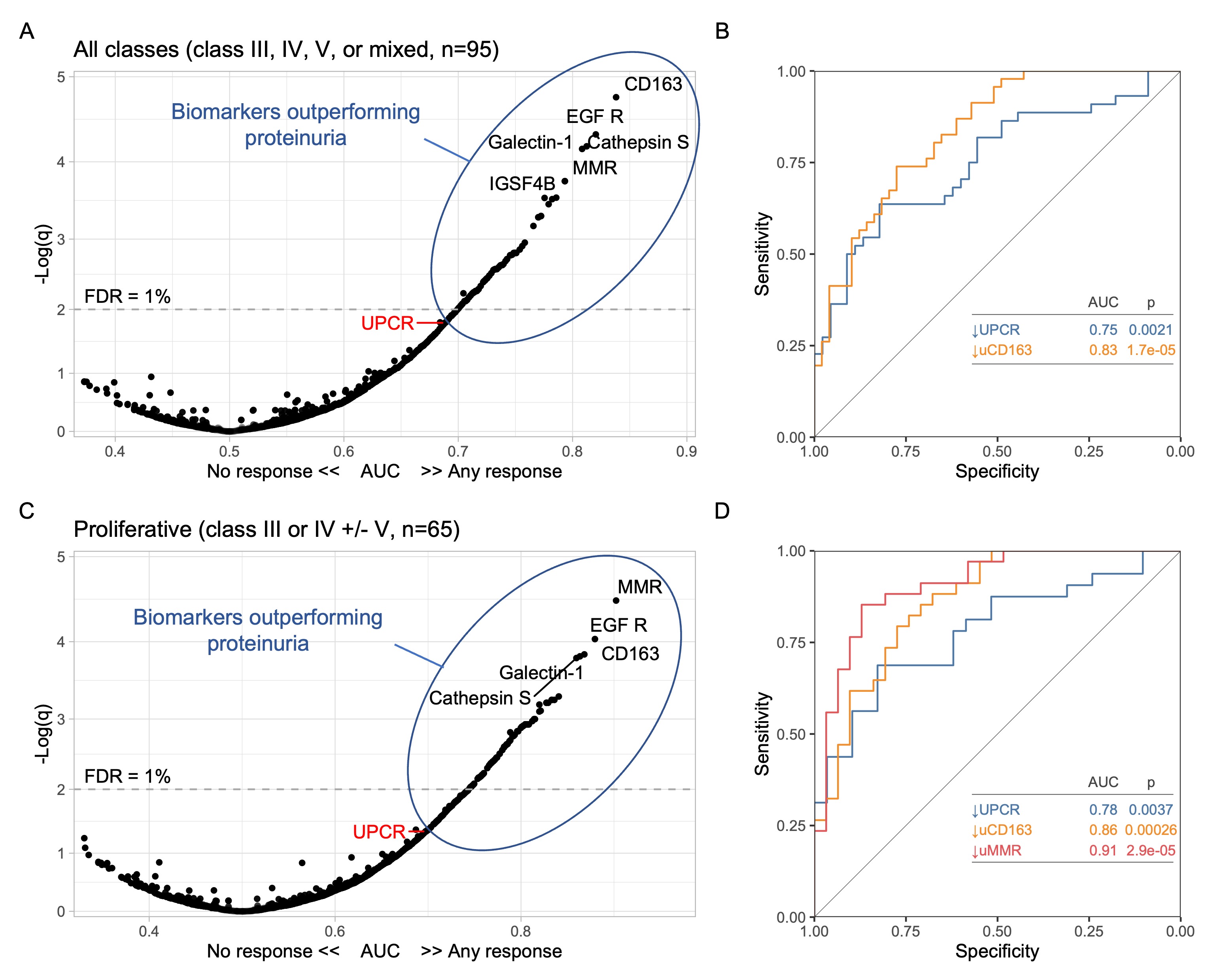 Figure 2. Changes in specific urinary biomarkers at 3 months predict 1-year response better than proteinuria (urine protein-to-creatinine ratio, UPCR). Analyses for all patients and for proliferative LN only are summarized and A-B and C-D, respectively. (A, C) Volcano plots displaying the area under the curve (AUC) indicating the performance of a change of a biomarker at 3 months compared to the urine sample collected at time of kidney biopsy. For reference, UPCR (the current clinically used biomarker) is indicated in red. (B, D) ROC curves comparing the performance of a decline at 3 months of UPCR and urinary biomarkers. FDR, false discovery rate. q, adjusted p value.
Figure 2. Changes in specific urinary biomarkers at 3 months predict 1-year response better than proteinuria (urine protein-to-creatinine ratio, UPCR). Analyses for all patients and for proliferative LN only are summarized and A-B and C-D, respectively. (A, C) Volcano plots displaying the area under the curve (AUC) indicating the performance of a change of a biomarker at 3 months compared to the urine sample collected at time of kidney biopsy. For reference, UPCR (the current clinically used biomarker) is indicated in red. (B, D) ROC curves comparing the performance of a decline at 3 months of UPCR and urinary biomarkers. FDR, false discovery rate. q, adjusted p value.
Disclosures: A. Fava, Sanofi; L. Magder, None; D. Goldman, None; J. Buyon, None; B. Diamond, None; J. Guthridge, None; W. Apruzzese, None; D. Fine, None; J. Monroy-Trujillo, None; M. Atta, Kira, Horizon, Bristol-Myers Squibb(BMS), REATA, Morphosys AG, Sentien; P. Izmirly, Momenta/Janssen; H. Belmont, None; A. Davidson, None; M. Dall'Era, None; D. Rao, Janssen, Merck, Bristol-Myers Squibb, Scipher Medicine, HiFiBio, Inc., AstraZeneca, Pfizer; A. Arazi, None; N. Hacohen, DangerBio/Related Sciences; S. Raychaudhuri, Mestag, Inc, Rheos Medicines, Janssen, Pfizer, Biogen; t. (AMP) RA/SLE, None; M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee.
Background/Purpose: A decline of urine protein-to-creatinine ratio (UPCR) to < 0.5 is associated with better long-term preservation of kidney function in lupus nephritis (LN). UPCR < 0.5 defines complete response in guidelines and clinical trials when achieved after 1 or 2 years. Biomarkers of early response are needed to guide early treatment changes. We studied longitudinal urine proteomic profiles in LN to identify early predictors of proteinuric response.
Methods: We quantified 1200 biomarkers (Kiloplex, RayBiotech) in urine samples collected on the day of (73%) or within 3 weeks (27%) of kidney biopsy and week 12, 24, or 52 in LN patients (ISN class III, IV, V, or mixed) with proteinuria > 1 g/d. Response was defined at one year from renal biopsy: Complete = UPCR < 0.5, serum creatinine (sCr) < 125% of baseline, prednisone ≤ 10mg/d; Partial = UPCR < 50% from baseline but >0.5, sCr < 125% of baseline, but prednisone allowed to 15 mg/d; Non responder = not meeting previous definitions.
Results: A total of 127 patients were included: 48 (38%) with pure proliferative LN (class III or IV), 41 (32%) with mixed LN (III or IV +/- V), and 38% (30%) with pure membranous LN. Response was complete in 34 (27%), partial in 29 (23%), and none in 64 (50%). There were no urinary biomarkers at baseline that predicted response. We then analyzed the changes in urinary proteins at 3 months compared to baseline. Patients who responded at 1 year showed an early decline in 51 urinary proteins led by CD163, IL-16, and macrophage mannose receptor (MMR, CD206) (Figure 1A) which matched the proteomic signature associated with histological activity (Figure 1B). No changes were observed in nonresponders. The decline of several urinary biomarkers at 3 months outperformed a decline in UPCR (clinical standard) in predicting the 1 year response (Figure 2A). In particular, a decline of CD163 predicted 1 year response in ROC analysis with an area under the curve (AUC) of 83% compared to an AUC of 75% for UPCR decline (Figure 2B). In proliferative LN, urinary biomarkers displayed superior performance with an AUC of 91%, 86%, and 78% for the decline of MMR, CD163, and UPCR, respectively (Figure 2C-D). Pathway enrichment analysis identified leukocyte activation, neutrophil degranulation, and matrix degradation as the main pathways reduced at 3 months in responders.
Conclusion: An early decline in urinary biomarkers of histological activity is associated with proteinuric response at 1 year. These findings indicate that effective immunosuppression induces by three months an immunological response in the kidney that can be noninvasively monitored in the urine. Biomarkers of immunological response outperformed early decline of UPCR, the standard of care, in predicting 1-year proteinuric response, especially in proliferative LN. Because biomarkers of immunological response parallel intrarenal activity, they could detect early treatment response/failure and allow early treatment changes. They could serve as surrogate endpoints in clinical trials. Longitudinal studies are needed to confirm that this immunological response is a better predictor of long-term kidney function preservation than proteinuric responses.
 Figure 1. Proteins linked to intrarenal activity (NIH activity index) decrease in treatment responders. Volcano plot of the changes of the urinary proteomic profile of treatment responders at 3 months (A) after kidney biopsy/treatment compared to baseline at time of biopsy. (B) Volcano plot displaying the Spearman’s correlation coefficients of urinary biomarkers at time of biopsy with histological activity defined as the NIH activity index. Blue arrows indicate 4 examples of shared urinary biomarkers that indicate both intrarenal activity and response to treatment. FDR, false discovery rate; q, adjusted p value.
Figure 1. Proteins linked to intrarenal activity (NIH activity index) decrease in treatment responders. Volcano plot of the changes of the urinary proteomic profile of treatment responders at 3 months (A) after kidney biopsy/treatment compared to baseline at time of biopsy. (B) Volcano plot displaying the Spearman’s correlation coefficients of urinary biomarkers at time of biopsy with histological activity defined as the NIH activity index. Blue arrows indicate 4 examples of shared urinary biomarkers that indicate both intrarenal activity and response to treatment. FDR, false discovery rate; q, adjusted p value. Figure 2. Changes in specific urinary biomarkers at 3 months predict 1-year response better than proteinuria (urine protein-to-creatinine ratio, UPCR). Analyses for all patients and for proliferative LN only are summarized and A-B and C-D, respectively. (A, C) Volcano plots displaying the area under the curve (AUC) indicating the performance of a change of a biomarker at 3 months compared to the urine sample collected at time of kidney biopsy. For reference, UPCR (the current clinically used biomarker) is indicated in red. (B, D) ROC curves comparing the performance of a decline at 3 months of UPCR and urinary biomarkers. FDR, false discovery rate. q, adjusted p value.
Figure 2. Changes in specific urinary biomarkers at 3 months predict 1-year response better than proteinuria (urine protein-to-creatinine ratio, UPCR). Analyses for all patients and for proliferative LN only are summarized and A-B and C-D, respectively. (A, C) Volcano plots displaying the area under the curve (AUC) indicating the performance of a change of a biomarker at 3 months compared to the urine sample collected at time of kidney biopsy. For reference, UPCR (the current clinically used biomarker) is indicated in red. (B, D) ROC curves comparing the performance of a decline at 3 months of UPCR and urinary biomarkers. FDR, false discovery rate. q, adjusted p value.Disclosures: A. Fava, Sanofi; L. Magder, None; D. Goldman, None; J. Buyon, None; B. Diamond, None; J. Guthridge, None; W. Apruzzese, None; D. Fine, None; J. Monroy-Trujillo, None; M. Atta, Kira, Horizon, Bristol-Myers Squibb(BMS), REATA, Morphosys AG, Sentien; P. Izmirly, Momenta/Janssen; H. Belmont, None; A. Davidson, None; M. Dall'Era, None; D. Rao, Janssen, Merck, Bristol-Myers Squibb, Scipher Medicine, HiFiBio, Inc., AstraZeneca, Pfizer; A. Arazi, None; N. Hacohen, DangerBio/Related Sciences; S. Raychaudhuri, Mestag, Inc, Rheos Medicines, Janssen, Pfizer, Biogen; t. (AMP) RA/SLE, None; M. Petri, Exagen, AstraZeneca, Alexion, Amgen, AnaptysBio, Argenx, Aurinia, Biogen, Caribou Biosciences, CVS Health, EMD Serono, Eli Lilly, Emergent Biosolutions, GlaxoSmithKline (GSK), IQVIA, Janssen, Kira Pharmaceuticals, MedShr, Sanofi, SinoMab, Thermofisher, BPR Scientific Advisory Committee.

