Back
Abstract Session
Systemic lupus erythematosus (SLE)
Session: Abstracts: SLE – Diagnosis, Manifestations, and Outcomes I: Renal Aspects (0536–0541)
0537: Neutrophil Extracellular Traps as a Biomarker to Predict Outcomes in Lupus Nephritis
Saturday, November 12, 2022
4:45 PM – 4:55 PM Eastern Time
Location: Room 122
- LW
Laura Whittall Garcia, MD
University Health Network
Toronto, ON, Canada
Presenting Author(s)
Laura Patricia Whittall Garcia1, Farnoosh Naderinavi1, Zahi Touma2, Dafna Gladman3, Murray Urowitz4, Anna Konvalinka1 and Joan Wither5, 1University Health Network, Toronto, ON, Canada, 2Schroeder Arthritis Institute, Krembil Research Institute, University Health Network and University of Toronto, Toronto, ON, Canada, 3Toronto Western Hospital, Schroeder Arthritis Institute, Toronto, ON, Canada, 4University of Toronto, University Health Network, Schroeder Arthritis Institute, Toronto, ON, Canada, 5Schroeder Arthritis Institute, Krembil Research Institute, University Health Network, University of Toronto, Toronto, ON, Canada
Background/Purpose: Neutrophil Extracellular Traps (NETs) have been implicated in Lupus Nephritis (LN) pathogenesis. SLE neutrophils release High Mobility Group Box-1 (HMGB1) protein, in turn, HMGB1 in NETs correlates with histologic findings of Active LN (ALN). The aim was to determine if the amount of NET complexes (Elastase-DNA and HMGB1-DNA) in serum at the time of a LN flare predicts renal outcomes in the following 24 months.
Methods: The study had a 2-staged approach. In an exploratory cohort composed of active SLE (clinical SLEDAI ≥ 1), inactive SLE and healthy controls (HC), we assessed the association between our in-house ELISA assays for Elastase-DNA and HMGB1-DNA complexes and ALN. A separate LN cohort was then used to determine the utility of NET complexes to predict renal outcomes over the subsequent 24 months. All patients had ALN, defined as a 24-hour urine protein >500mg with a subsequent modification in therapy by the treating physician, a baseline eGFR >30ml/min (3 months prior to the flare), stored serum sample ±3 months from the renal flare, and at least 2-years follow-up. The following outcomes were ascertained: Complete response (CR) at 12 and 24 months after flare (proteinuria < 500mg/day and a serum creatinine within 15% of the baseline); severe renal impairment (eGFR≤30ml/min) at 12 and 24 months after flare; and the percentage decline in the eGFR over the 24 months after flare.
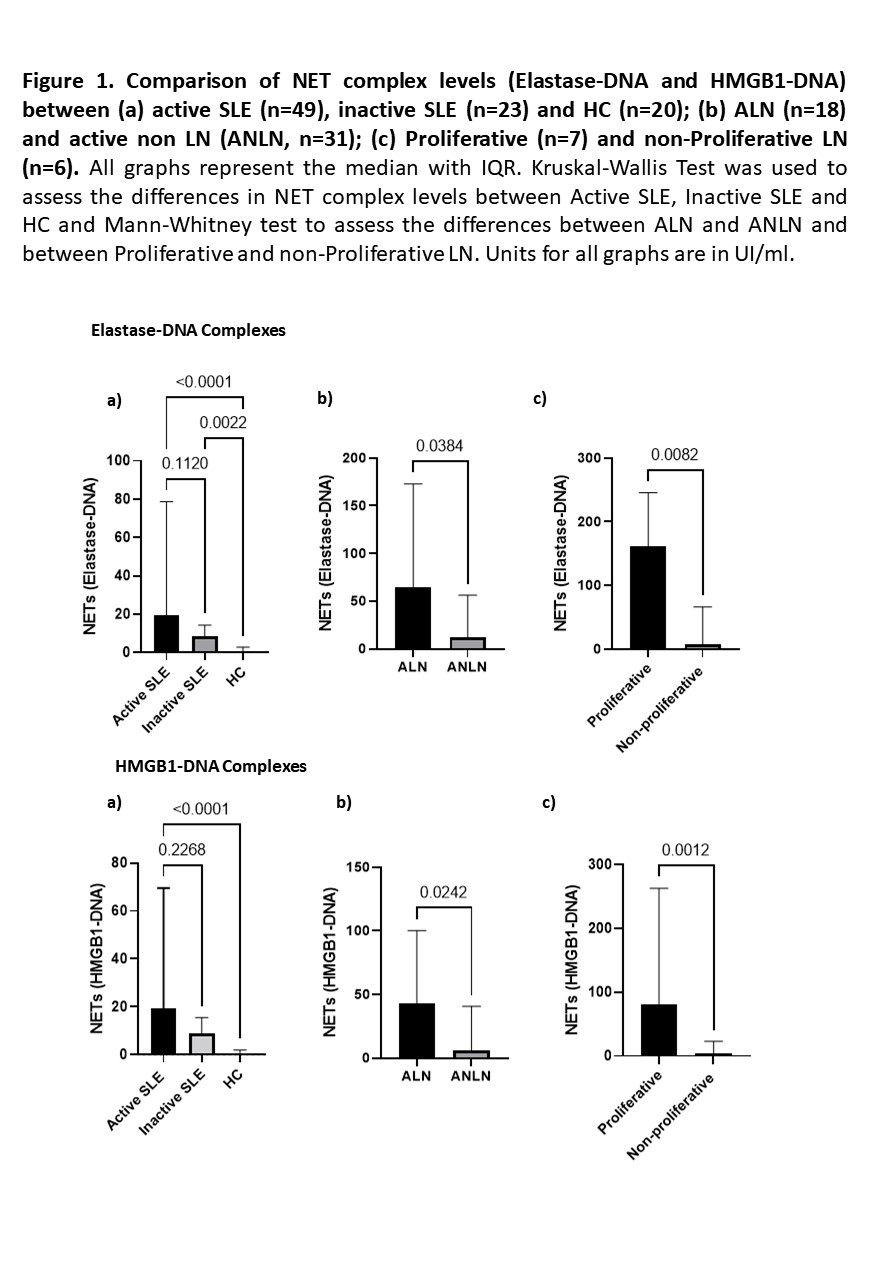
Results: Ninety-two individuals were included in the exploratory cohort (49 active SLE, 23 inactive SLE and 20 HC). NET complexes were significantly higher in SLE patients compared to HC and tended to be higher in active SLE compared to inactive patients. Patients with ALN (36.7%) had significantly higher levels of NET complexes compared to active SLE without LN. Furthermore, patients with proliferative LN had higher levels of NET complexes compared to non-proliferative LN (Figure 1).
The LN cohort included 109 ALN patients. The median (IQR) age was 29 (23-41) years, 84% were women, and disease duration was 6.4 (0.8-10.5) years. 37.9% were Caucasian, 22.2% Black and 17.5% Asian, the baseline eGFR was 112 (97-127) ml/min. 77.9% had a kidney biopsy at the time of the LN flare, of whom 55.9% had a proliferative or mixed class, 17.4% class V, and 4.5% class I or II. 39.4% and 50.5% of the ALN patients achieved CR at 12 and 24 months, respectively and 11% had an eGFR ≤ 30ml/min after 24 months.
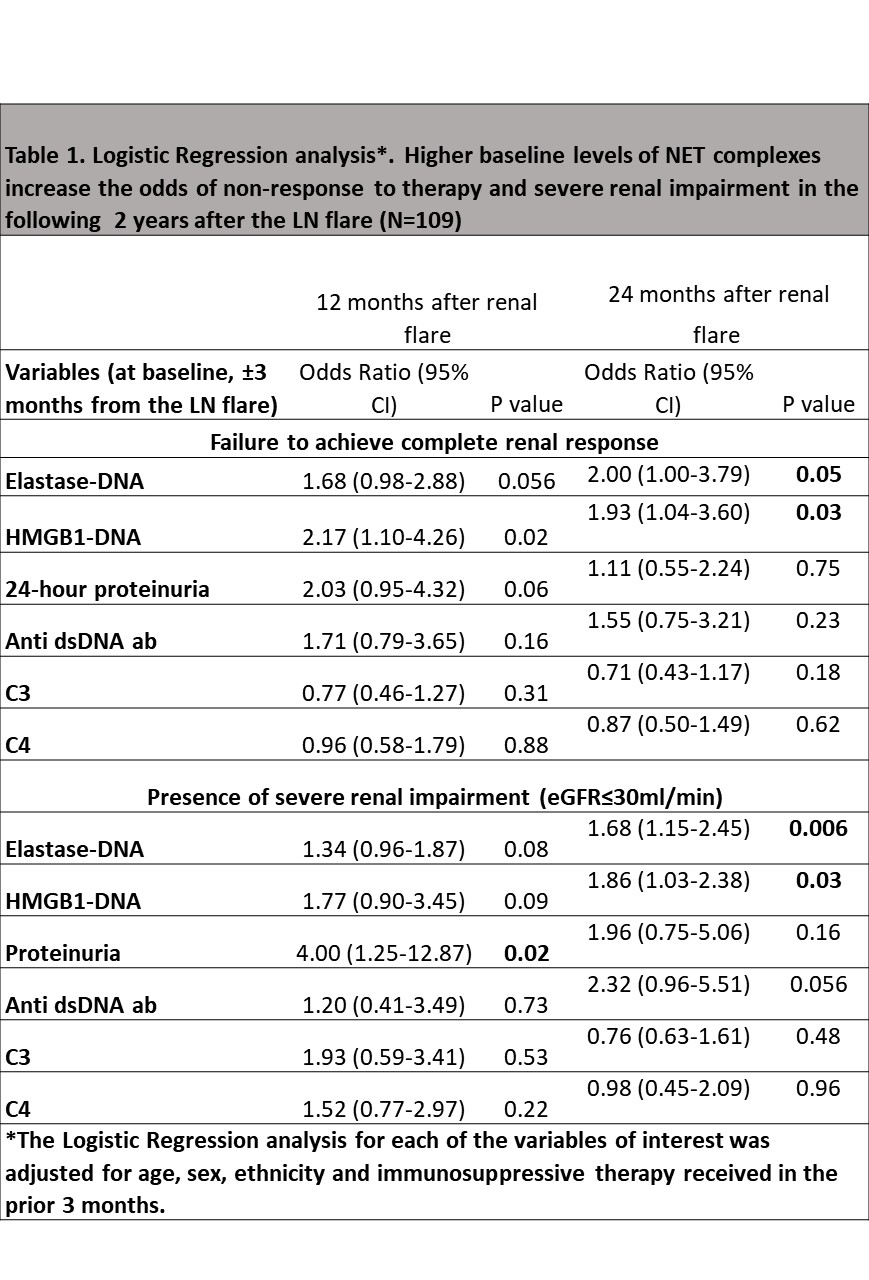
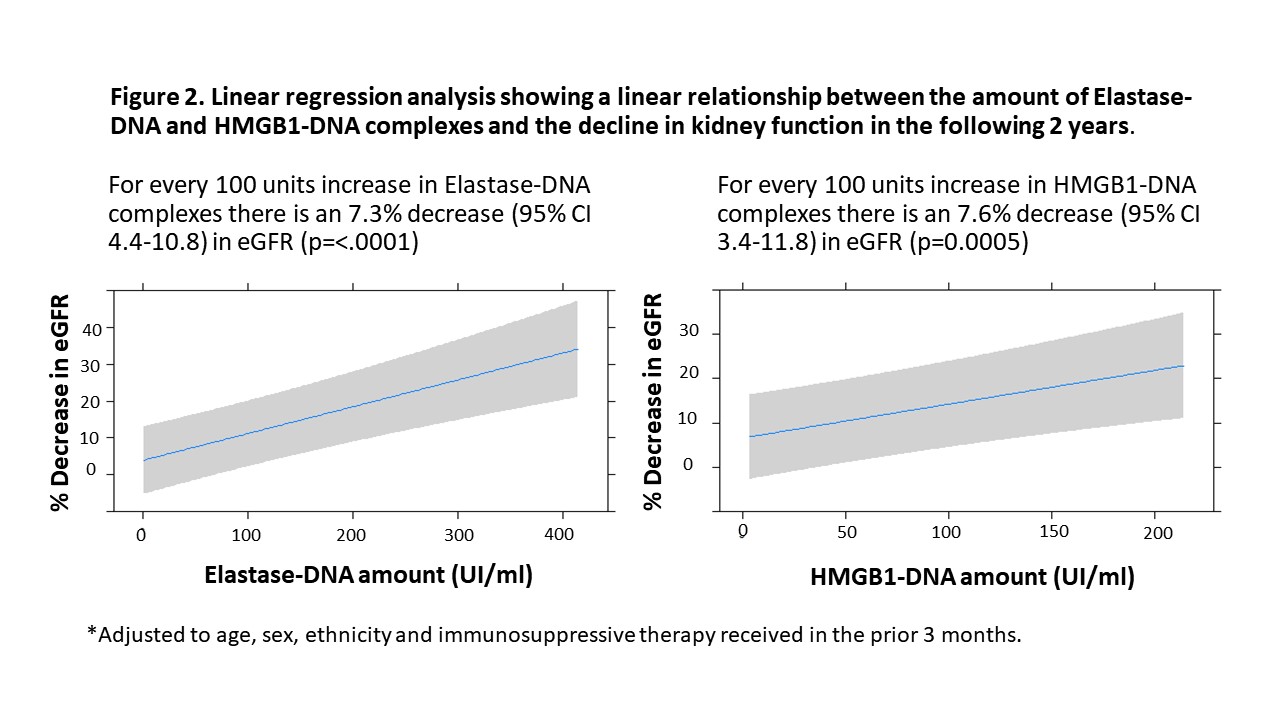
Similar to the results from the exploratory cohort, proliferative LN had higher levels of NET complexes compared to non-proliferative LN patients (Elastase-DNA: 111.7 vs 25.9, p=0.0003; HMGB1-DNA: 85.2 vs 25.4, p=0.002, proliferative vs non-proliferative, respectively). Patients with higher baseline levels of NET complexes had higher odds of not achieving CR and of having severe renal impairment after 24 months of the flare. NET complexes outperformed conventional biomarkers (Table 1). There was a linear relationship between the amount of baseline Elastase-DNA and HMGB1-DNA complexes and the decline in renal function in the subsequent 24 months (Figure 2).
Conclusion: Elastase-DNA and HMGB1-DNA complexes predicted renal outcomes, including response to therapy and decline in kidney function at 2 years after the LN flare.
Disclosures: L. Whittall Garcia, None; F. Naderinavi, None; Z. Touma, None; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; M. Urowitz, None; A. Konvalinka, None; J. Wither, AstraZeneca, Pfizer.
Background/Purpose: Neutrophil Extracellular Traps (NETs) have been implicated in Lupus Nephritis (LN) pathogenesis. SLE neutrophils release High Mobility Group Box-1 (HMGB1) protein, in turn, HMGB1 in NETs correlates with histologic findings of Active LN (ALN). The aim was to determine if the amount of NET complexes (Elastase-DNA and HMGB1-DNA) in serum at the time of a LN flare predicts renal outcomes in the following 24 months.
Methods: The study had a 2-staged approach. In an exploratory cohort composed of active SLE (clinical SLEDAI ≥ 1), inactive SLE and healthy controls (HC), we assessed the association between our in-house ELISA assays for Elastase-DNA and HMGB1-DNA complexes and ALN. A separate LN cohort was then used to determine the utility of NET complexes to predict renal outcomes over the subsequent 24 months. All patients had ALN, defined as a 24-hour urine protein >500mg with a subsequent modification in therapy by the treating physician, a baseline eGFR >30ml/min (3 months prior to the flare), stored serum sample ±3 months from the renal flare, and at least 2-years follow-up. The following outcomes were ascertained: Complete response (CR) at 12 and 24 months after flare (proteinuria < 500mg/day and a serum creatinine within 15% of the baseline); severe renal impairment (eGFR≤30ml/min) at 12 and 24 months after flare; and the percentage decline in the eGFR over the 24 months after flare.
Results: Ninety-two individuals were included in the exploratory cohort (49 active SLE, 23 inactive SLE and 20 HC). NET complexes were significantly higher in SLE patients compared to HC and tended to be higher in active SLE compared to inactive patients. Patients with ALN (36.7%) had significantly higher levels of NET complexes compared to active SLE without LN. Furthermore, patients with proliferative LN had higher levels of NET complexes compared to non-proliferative LN (Figure 1).
The LN cohort included 109 ALN patients. The median (IQR) age was 29 (23-41) years, 84% were women, and disease duration was 6.4 (0.8-10.5) years. 37.9% were Caucasian, 22.2% Black and 17.5% Asian, the baseline eGFR was 112 (97-127) ml/min. 77.9% had a kidney biopsy at the time of the LN flare, of whom 55.9% had a proliferative or mixed class, 17.4% class V, and 4.5% class I or II. 39.4% and 50.5% of the ALN patients achieved CR at 12 and 24 months, respectively and 11% had an eGFR ≤ 30ml/min after 24 months.
Similar to the results from the exploratory cohort, proliferative LN had higher levels of NET complexes compared to non-proliferative LN patients (Elastase-DNA: 111.7 vs 25.9, p=0.0003; HMGB1-DNA: 85.2 vs 25.4, p=0.002, proliferative vs non-proliferative, respectively). Patients with higher baseline levels of NET complexes had higher odds of not achieving CR and of having severe renal impairment after 24 months of the flare. NET complexes outperformed conventional biomarkers (Table 1). There was a linear relationship between the amount of baseline Elastase-DNA and HMGB1-DNA complexes and the decline in renal function in the subsequent 24 months (Figure 2).
Conclusion: Elastase-DNA and HMGB1-DNA complexes predicted renal outcomes, including response to therapy and decline in kidney function at 2 years after the LN flare.



Disclosures: L. Whittall Garcia, None; F. Naderinavi, None; Z. Touma, None; D. Gladman, AbbVie, Amgen, Eli Lilly, Janssen, Gilead, Novartis, Pfizer, Bristol-Myers Squibb(BMS), Galapagos, UCB Pharma, Celgene; M. Urowitz, None; A. Konvalinka, None; J. Wither, AstraZeneca, Pfizer.

