Back
Poster Session D
Systemic lupus erythematosus (SLE)
Session: (1707–1726) SLE – Animal Models Poster
1726: Impaired Dynamic X-Chromosome Inactivation Maintenance in T Lymphocytes Is a Feature of Spontaneous Lupus in Female Mice and Is Exacerbated in Female-Biased Disease Models
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- NJ
Nikhil Jiwrajka, MD
Hospital of the University of Pennsylvania
Philadelphia, PA, United States
Abstract Poster Presenter(s)
Nikhil Jiwrajka1, Natalie Toothacre2, Zachary Beethem2, Sarah Sting2, Katherine Forsyth2, Amanda Driscoll2, William Stohl3 and Montserrat Anguera2, 1Division of Rheumatology, Department of Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA, 2Department of Biomedical Sciences, University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA, 3University of Southern California, Los Angeles, CA
Background/Purpose: SLE is highly female-biased, yet the molecular origins of this bias remain unclear. The X chromosome contains many immune-related genes, suggesting that X-linked epigenetic dysregulation may contribute to female-biased immune responses. X-Chromosome Inactivation (XCI) is an X-chromosome-specific epigenetic regulatory mechanism that equalizes X-linked gene dosage between XX females and XY males. It is initiated and maintained by the non-coding RNA Xist, which together with various heterochromatic marks, 'coats' the inactive X (Xi) to effect transcriptional silencing. B and T cells uniquely exhibit dynamic XCI maintenance (dXCIm), in which naïve cells lack Xist RNA localization at the Xi despite Xist transcription. Upon cellular activation, Xist RNA and heterochromatic marks dynamically relocalize to the Xi. dXCIm is impaired in lymphocytes from the female-biased NZB/W F1 mouse model of spontaneous SLE, specifically at the late stages of disease. Using the female-biased NZM2328 and the sex-neutral MRL/lpr mouse models of spontaneous SLE, we asked whether impaired dXCIm is observed in other models of spontaneous SLE and whether it is specific to female-biased disease.
Methods: CD23+ B cells and CD3+ T cells were purified from age-matched wild-type (WT) C57BL/6, MRL/lpr, and NZM2328 female mice at three timepoints via sequential positive and negative splenocyte selection (Figure 1). B cells were activated with CpG for 24 hours and T cells with anti-CD3/CD28 for 48 hours, and both time 0 and activated cells were processed for sequential Xist RNA FISH, H3K27me3 IF, and RNAseq. Xist localization scores and H3K27me3 foci were quantified by one of two investigators based on the assessment of at least 100 nuclei per biological replicate.
Results: Xist RNA relocalization was impaired in activated T cells from the non-female-biased MRL/lpr model (p < 0.05 vs. WT) and was markedly impaired in activated T cells from the female-biased NZM2328 model (p < 0.001 vs WT, p < 0.05 vs. MRL/lpr) (Figure 2). The percentage of T cells with H3K27me3 foci was also decreased in both MRL/lpr and NZM2328 females relative to WT female mice (p < 0.001). Conversely, Xist RNA localization and the percentage of cells with H3K27me3 foci were similar amongst activated B cells from WT, MRL/lpr, and NZM2328 female mice. While impairment of Xist RNA relocalization in T cells from MRL/lpr mice was only evident at the onset of clinical disease, impairment in NZM2328 mice was observed throughout the lifespan, before the onset of clinical disease. RNAseq of activated T cells from NZM2328 females revealed an upregulation of immune-related X-linked genes relative to male mice, as well as a significant downregulation of genes known to interact with Xist RNA (Figure 3).
Conclusion: Impaired dXCIm in lymphocytes is not specific to female-biased models of spontaneous SLE; however, the chronic, exaggerated impairment observed in T cells from the female-biased NZM2328 model suggests that abnormal dXCIm likely contributes to sex-biased disease. Given our prior findings in the female-biased NZB/W F1 model, our data suggest that abnormal dXCIm may confer female bias through diverse mechanisms involving either T cells, B cells, or both, depending on the genetic context.
.jpg) Figure 1. Schematic of the experimental approach and the concept of dynamic XCI maintenance in lymphocytes. A. CD3+ T and CD23+ B cells were isolated from the spleens of wild-type (WT), MRL/lpr, and NZM2328 female mice between 8 to 34 weeks of age. Purified primary B and T cells were subsequently activated in vitro with CpG or anti-CD3/CD28, respectively, and processed for sequential Xist RNA FISH and H3K27me3 IF. B. In dynamic XCI maintenance, resting lymphocytes from WT mice paradoxically lack the canonical Xist RNA “cloud”, which reappears upon cellular activation in association with the heterochromatic mark, H3K27me3.
Figure 1. Schematic of the experimental approach and the concept of dynamic XCI maintenance in lymphocytes. A. CD3+ T and CD23+ B cells were isolated from the spleens of wild-type (WT), MRL/lpr, and NZM2328 female mice between 8 to 34 weeks of age. Purified primary B and T cells were subsequently activated in vitro with CpG or anti-CD3/CD28, respectively, and processed for sequential Xist RNA FISH and H3K27me3 IF. B. In dynamic XCI maintenance, resting lymphocytes from WT mice paradoxically lack the canonical Xist RNA “cloud”, which reappears upon cellular activation in association with the heterochromatic mark, H3K27me3.
.jpg) Figure 2. Dynamic relocalization of Xist RNA and H3K27me3 is impaired in T cells, but not in B cells, from two mouse models of spontaneous lupus, with greater impairment in the female-biased disease model. A. The mean Xist localization score (1 = dispersed Xist RNA; 4 = robustly localized Xist RNA) ± SD is depicted for time 0 (open) and in vitro activated (filled) CD3+ T cells (triangles) and CD23+ B Cells (circles) from 8-34-week-old age-matched WT (green), MRL/lpr (orange), and NZM2328 (red) female mice. B. Proportion of splenic lymphocytes with H3K27me3 foci. C. Representative high-powered fields of sequential Xist RNA FISH and H3K27me3 IF. D. Linear regression of the Xist RNA localization scores in activated CD3+ T cells as a function of each mouse’s age, shaded based on the 95% confidence interval of the mean Xist RNA localization score. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001 by one-way ANOVA with Dunnett’s T3 correction for multiple comparisons.
Figure 2. Dynamic relocalization of Xist RNA and H3K27me3 is impaired in T cells, but not in B cells, from two mouse models of spontaneous lupus, with greater impairment in the female-biased disease model. A. The mean Xist localization score (1 = dispersed Xist RNA; 4 = robustly localized Xist RNA) ± SD is depicted for time 0 (open) and in vitro activated (filled) CD3+ T cells (triangles) and CD23+ B Cells (circles) from 8-34-week-old age-matched WT (green), MRL/lpr (orange), and NZM2328 (red) female mice. B. Proportion of splenic lymphocytes with H3K27me3 foci. C. Representative high-powered fields of sequential Xist RNA FISH and H3K27me3 IF. D. Linear regression of the Xist RNA localization scores in activated CD3+ T cells as a function of each mouse’s age, shaded based on the 95% confidence interval of the mean Xist RNA localization score. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001 by one-way ANOVA with Dunnett’s T3 correction for multiple comparisons.
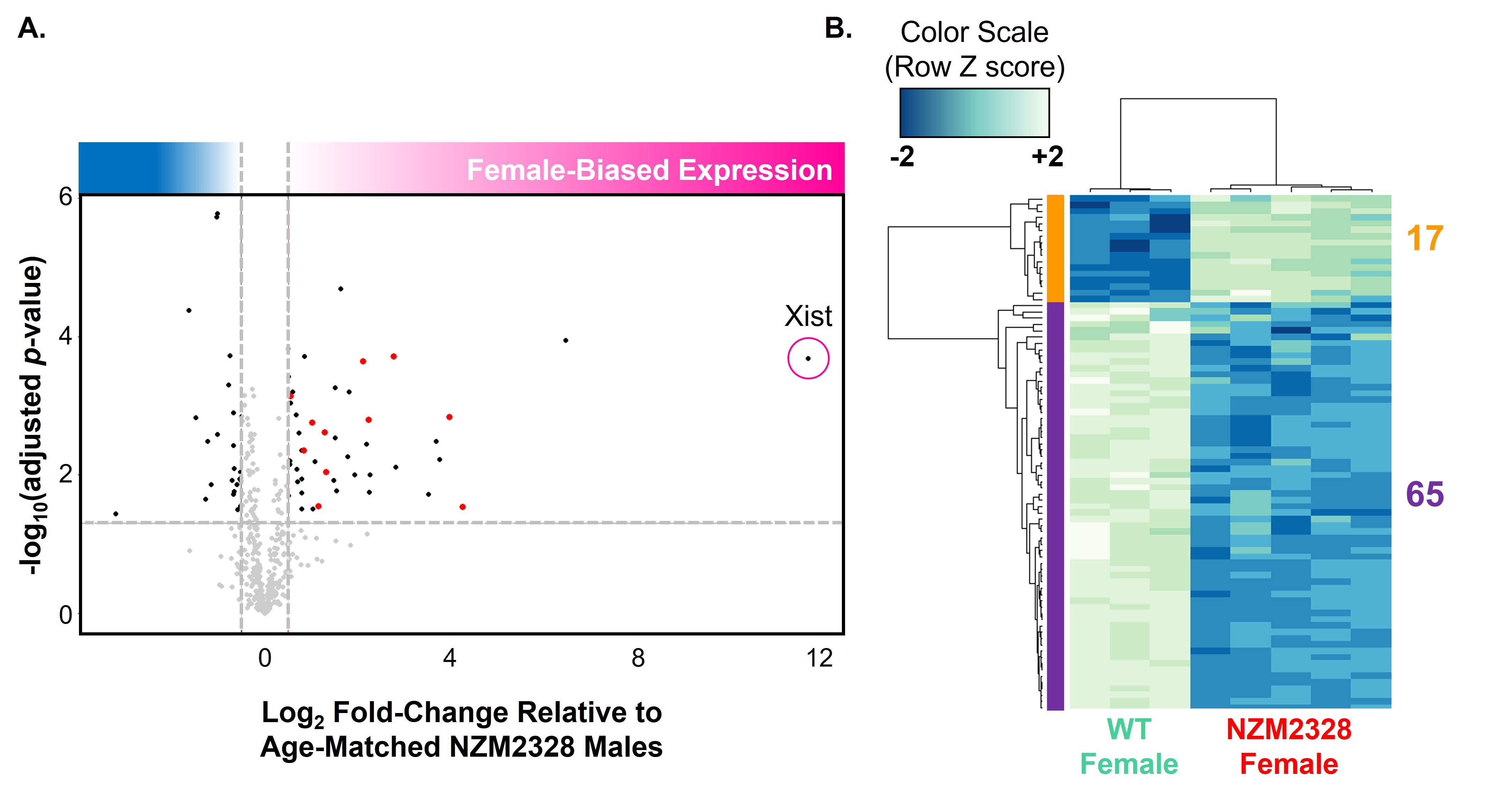 Figure 3. RNAseq of activated CD3+ T cells in NZM2328 females reveals an upregulation of several X-linked immune-related genes relative to NZM2328 males, and a significant downregulation of Xist-interacting genes. A. RNAseq of activated CD3+ T cells from 18-23-week-old NZM2328 females (n=5) and age-matched NZM2328 males (n=3) reveals 63 differentially expressed X-linked genes, 34 of which are upregulated with log2-fold change > 0.5 and Benjamini-Hochberg adjusted p-value < 0.05. Upregulated X-linked genes are enriched for immune functions (highlighted in red). B. RNAseq of activated CD3+ T cells from 18-23-week-old NZM2328 females (n=5) and age-matched WT females (n=3) reveals differential expression of 82 genes known to interact with Xist RNA, the majority of which are downregulated in the NZM2328 model.
Figure 3. RNAseq of activated CD3+ T cells in NZM2328 females reveals an upregulation of several X-linked immune-related genes relative to NZM2328 males, and a significant downregulation of Xist-interacting genes. A. RNAseq of activated CD3+ T cells from 18-23-week-old NZM2328 females (n=5) and age-matched NZM2328 males (n=3) reveals 63 differentially expressed X-linked genes, 34 of which are upregulated with log2-fold change > 0.5 and Benjamini-Hochberg adjusted p-value < 0.05. Upregulated X-linked genes are enriched for immune functions (highlighted in red). B. RNAseq of activated CD3+ T cells from 18-23-week-old NZM2328 females (n=5) and age-matched WT females (n=3) reveals differential expression of 82 genes known to interact with Xist RNA, the majority of which are downregulated in the NZM2328 model.
Disclosures: N. Jiwrajka, None; N. Toothacre, None; Z. Beethem, None; S. Sting, None; K. Forsyth, None; A. Driscoll, None; W. Stohl, None; M. Anguera, None.
Background/Purpose: SLE is highly female-biased, yet the molecular origins of this bias remain unclear. The X chromosome contains many immune-related genes, suggesting that X-linked epigenetic dysregulation may contribute to female-biased immune responses. X-Chromosome Inactivation (XCI) is an X-chromosome-specific epigenetic regulatory mechanism that equalizes X-linked gene dosage between XX females and XY males. It is initiated and maintained by the non-coding RNA Xist, which together with various heterochromatic marks, 'coats' the inactive X (Xi) to effect transcriptional silencing. B and T cells uniquely exhibit dynamic XCI maintenance (dXCIm), in which naïve cells lack Xist RNA localization at the Xi despite Xist transcription. Upon cellular activation, Xist RNA and heterochromatic marks dynamically relocalize to the Xi. dXCIm is impaired in lymphocytes from the female-biased NZB/W F1 mouse model of spontaneous SLE, specifically at the late stages of disease. Using the female-biased NZM2328 and the sex-neutral MRL/lpr mouse models of spontaneous SLE, we asked whether impaired dXCIm is observed in other models of spontaneous SLE and whether it is specific to female-biased disease.
Methods: CD23+ B cells and CD3+ T cells were purified from age-matched wild-type (WT) C57BL/6, MRL/lpr, and NZM2328 female mice at three timepoints via sequential positive and negative splenocyte selection (Figure 1). B cells were activated with CpG for 24 hours and T cells with anti-CD3/CD28 for 48 hours, and both time 0 and activated cells were processed for sequential Xist RNA FISH, H3K27me3 IF, and RNAseq. Xist localization scores and H3K27me3 foci were quantified by one of two investigators based on the assessment of at least 100 nuclei per biological replicate.
Results: Xist RNA relocalization was impaired in activated T cells from the non-female-biased MRL/lpr model (p < 0.05 vs. WT) and was markedly impaired in activated T cells from the female-biased NZM2328 model (p < 0.001 vs WT, p < 0.05 vs. MRL/lpr) (Figure 2). The percentage of T cells with H3K27me3 foci was also decreased in both MRL/lpr and NZM2328 females relative to WT female mice (p < 0.001). Conversely, Xist RNA localization and the percentage of cells with H3K27me3 foci were similar amongst activated B cells from WT, MRL/lpr, and NZM2328 female mice. While impairment of Xist RNA relocalization in T cells from MRL/lpr mice was only evident at the onset of clinical disease, impairment in NZM2328 mice was observed throughout the lifespan, before the onset of clinical disease. RNAseq of activated T cells from NZM2328 females revealed an upregulation of immune-related X-linked genes relative to male mice, as well as a significant downregulation of genes known to interact with Xist RNA (Figure 3).
Conclusion: Impaired dXCIm in lymphocytes is not specific to female-biased models of spontaneous SLE; however, the chronic, exaggerated impairment observed in T cells from the female-biased NZM2328 model suggests that abnormal dXCIm likely contributes to sex-biased disease. Given our prior findings in the female-biased NZB/W F1 model, our data suggest that abnormal dXCIm may confer female bias through diverse mechanisms involving either T cells, B cells, or both, depending on the genetic context.
.jpg) Figure 1. Schematic of the experimental approach and the concept of dynamic XCI maintenance in lymphocytes. A. CD3+ T and CD23+ B cells were isolated from the spleens of wild-type (WT), MRL/lpr, and NZM2328 female mice between 8 to 34 weeks of age. Purified primary B and T cells were subsequently activated in vitro with CpG or anti-CD3/CD28, respectively, and processed for sequential Xist RNA FISH and H3K27me3 IF. B. In dynamic XCI maintenance, resting lymphocytes from WT mice paradoxically lack the canonical Xist RNA “cloud”, which reappears upon cellular activation in association with the heterochromatic mark, H3K27me3.
Figure 1. Schematic of the experimental approach and the concept of dynamic XCI maintenance in lymphocytes. A. CD3+ T and CD23+ B cells were isolated from the spleens of wild-type (WT), MRL/lpr, and NZM2328 female mice between 8 to 34 weeks of age. Purified primary B and T cells were subsequently activated in vitro with CpG or anti-CD3/CD28, respectively, and processed for sequential Xist RNA FISH and H3K27me3 IF. B. In dynamic XCI maintenance, resting lymphocytes from WT mice paradoxically lack the canonical Xist RNA “cloud”, which reappears upon cellular activation in association with the heterochromatic mark, H3K27me3. .jpg) Figure 2. Dynamic relocalization of Xist RNA and H3K27me3 is impaired in T cells, but not in B cells, from two mouse models of spontaneous lupus, with greater impairment in the female-biased disease model. A. The mean Xist localization score (1 = dispersed Xist RNA; 4 = robustly localized Xist RNA) ± SD is depicted for time 0 (open) and in vitro activated (filled) CD3+ T cells (triangles) and CD23+ B Cells (circles) from 8-34-week-old age-matched WT (green), MRL/lpr (orange), and NZM2328 (red) female mice. B. Proportion of splenic lymphocytes with H3K27me3 foci. C. Representative high-powered fields of sequential Xist RNA FISH and H3K27me3 IF. D. Linear regression of the Xist RNA localization scores in activated CD3+ T cells as a function of each mouse’s age, shaded based on the 95% confidence interval of the mean Xist RNA localization score. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001 by one-way ANOVA with Dunnett’s T3 correction for multiple comparisons.
Figure 2. Dynamic relocalization of Xist RNA and H3K27me3 is impaired in T cells, but not in B cells, from two mouse models of spontaneous lupus, with greater impairment in the female-biased disease model. A. The mean Xist localization score (1 = dispersed Xist RNA; 4 = robustly localized Xist RNA) ± SD is depicted for time 0 (open) and in vitro activated (filled) CD3+ T cells (triangles) and CD23+ B Cells (circles) from 8-34-week-old age-matched WT (green), MRL/lpr (orange), and NZM2328 (red) female mice. B. Proportion of splenic lymphocytes with H3K27me3 foci. C. Representative high-powered fields of sequential Xist RNA FISH and H3K27me3 IF. D. Linear regression of the Xist RNA localization scores in activated CD3+ T cells as a function of each mouse’s age, shaded based on the 95% confidence interval of the mean Xist RNA localization score. * = p < 0.05, ** = p < 0.01, *** = p < 0.001, **** = p < 0.0001 by one-way ANOVA with Dunnett’s T3 correction for multiple comparisons. Figure 3. RNAseq of activated CD3+ T cells in NZM2328 females reveals an upregulation of several X-linked immune-related genes relative to NZM2328 males, and a significant downregulation of Xist-interacting genes. A. RNAseq of activated CD3+ T cells from 18-23-week-old NZM2328 females (n=5) and age-matched NZM2328 males (n=3) reveals 63 differentially expressed X-linked genes, 34 of which are upregulated with log2-fold change > 0.5 and Benjamini-Hochberg adjusted p-value < 0.05. Upregulated X-linked genes are enriched for immune functions (highlighted in red). B. RNAseq of activated CD3+ T cells from 18-23-week-old NZM2328 females (n=5) and age-matched WT females (n=3) reveals differential expression of 82 genes known to interact with Xist RNA, the majority of which are downregulated in the NZM2328 model.
Figure 3. RNAseq of activated CD3+ T cells in NZM2328 females reveals an upregulation of several X-linked immune-related genes relative to NZM2328 males, and a significant downregulation of Xist-interacting genes. A. RNAseq of activated CD3+ T cells from 18-23-week-old NZM2328 females (n=5) and age-matched NZM2328 males (n=3) reveals 63 differentially expressed X-linked genes, 34 of which are upregulated with log2-fold change > 0.5 and Benjamini-Hochberg adjusted p-value < 0.05. Upregulated X-linked genes are enriched for immune functions (highlighted in red). B. RNAseq of activated CD3+ T cells from 18-23-week-old NZM2328 females (n=5) and age-matched WT females (n=3) reveals differential expression of 82 genes known to interact with Xist RNA, the majority of which are downregulated in the NZM2328 model.Disclosures: N. Jiwrajka, None; N. Toothacre, None; Z. Beethem, None; S. Sting, None; K. Forsyth, None; A. Driscoll, None; W. Stohl, None; M. Anguera, None.

