Back
Abstract Session
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: Abstracts: Spondyloarthritis Including PsA – Treatment I: Axial Spondyloarthritis (0542–0547)
0545: Continuing (Full or Reduced Treatment) versus Withdrawing from Golimumab Treatment in Patients with Non-radiographic Spondylarthritis Who Achieved Inactive Disease: Efficacy and Safety Results from a Placebo-Controlled, Randomized Withdrawal and Retreatment Study (GO-BACK)
Saturday, November 12, 2022
5:15 PM – 5:25 PM Eastern Time
Location: Room 204
- CW
Cindy Weinstein, MD, PhD
Merck & Co, Inc
Rahway, NJ, United States
Presenting Author(s)
Cindy Weinstein1, Paula Sliwinska-Stanczyk2, Tomas Hala3, Marina Stanislav4, Anjela Tzontcheva5, Ruji Yao1, Yuliya Berd1, Sean Curtis1 and George Philip1, 1Merck & Co., Inc., Rahway, NJ, 2Reumatika Centrum Reumatologii, Warsaw, Poland, 3Center for Clinical and Basic Research, Pardubice, Czech Republic, 4Vladimirsky Moscow Regional Scientific Institution, Moscow, Russia, 5MSD Switzerland, Zürich, Switzerland
Background/Purpose: Non-radiographic axial spondyloarthrits (nr-axSpA) is a rheumatic disease that is predominantly associated with chronic back pain and stiffness.1 Results from the Phase III GO-AHEAD trial demonstrate the efficacy and safety of golimumab (GLM) in the treatment of nr-axSpA;2,3 however, the efficacy and safety of GLM withdrawal has not been studied. This study evaluates the effect of GLM withdrawal on the incidence of a flare in disease activity and to characterize clinical response after GLM retreatment for a disease flare.
Methods: This Phase-IV parallel-group withdrawal study (GO-BACK) included adult participants, aged 18-45 years, with active nr-axSpA for ≤ 5 years, chronic back pain of ≥ 3 months, objective signs of inflammation, and intolerance or inadequate response to non-steroid anti-inflammatory drugs. In Period 1, participants received open-label (OL) subcutaneous GLM monthly for up to 10 months. In Period 2, participants who achieved inactive disease were randomized 1:1:1 to either receive monthly placebo (PBO, treatment withdrawal), or continued GLM treatment given monthly (GLM QMT, full-treatment) or every 2-months (GLM Q2MT, reduced-treatment). Participants who did not have a disease flare continued randomized treatment for ~12 months, whereas participants with a disease flare discontinued randomized treatment and resumed monthly dosing with OL GLM. All participants were followed for safety until ~3 months after their last dose. The primary endpoint was the proportion of participants without a disease flare on continued GLM treatment (GLM QMT or GLM Q2MT) versus GLM withdrawal (PBO). Among participants randomized to GLM QMT or PBO who experienced a disease flare, clinical response to monthly GLM retreatment was evaluated as a secondary endpoint.
Results: Of the 323 Period 1 participants, 188 achieved inactive disease and were eligible for treatment withdrawal. At baseline, Period 2 participants had a median disease symptom duration of 8 y, a median age of 32.0 y, 70.4% were male and all were White. Continued GLM treatment (QMT or Q2MT) was superior to treatment withdrawal (p< 0.001), with a greater proportion of GLM QMT participants having no disease flares compared to GLM Q2MT (50.1% vs 34.4%) [Figure 1]. Similarly, the time to first flare was significantly longer (log-rank p < 0.0001) for participants continuing GLM treatment compared with the PBO group [Figure 2]. Of the 53 participants who had a confirmed disease flare, 51 (96.2%) attained a clinical response to GLM within the first 3 months of retreatment. Adverse events (AEs) were consistent with the known safety profile for GLM. Throughout the study, there were no deaths or serious drug-related adverse events (AEs). In Period 2, the incidences of serious AEs (1.6%) and non-serious drug-related AEs (5.8%) were low, and both were comparable across the treatment groups.
Conclusion: Among participants with active nr-axSpA who attained inactive disease after 10 months of GLM treatment, continued GLM treatment provides superior protection against disease flares compared to GLM withdrawal. GLM was generally safe and well-tolerated, with a comparable incidence of AEs across all treatment groups.
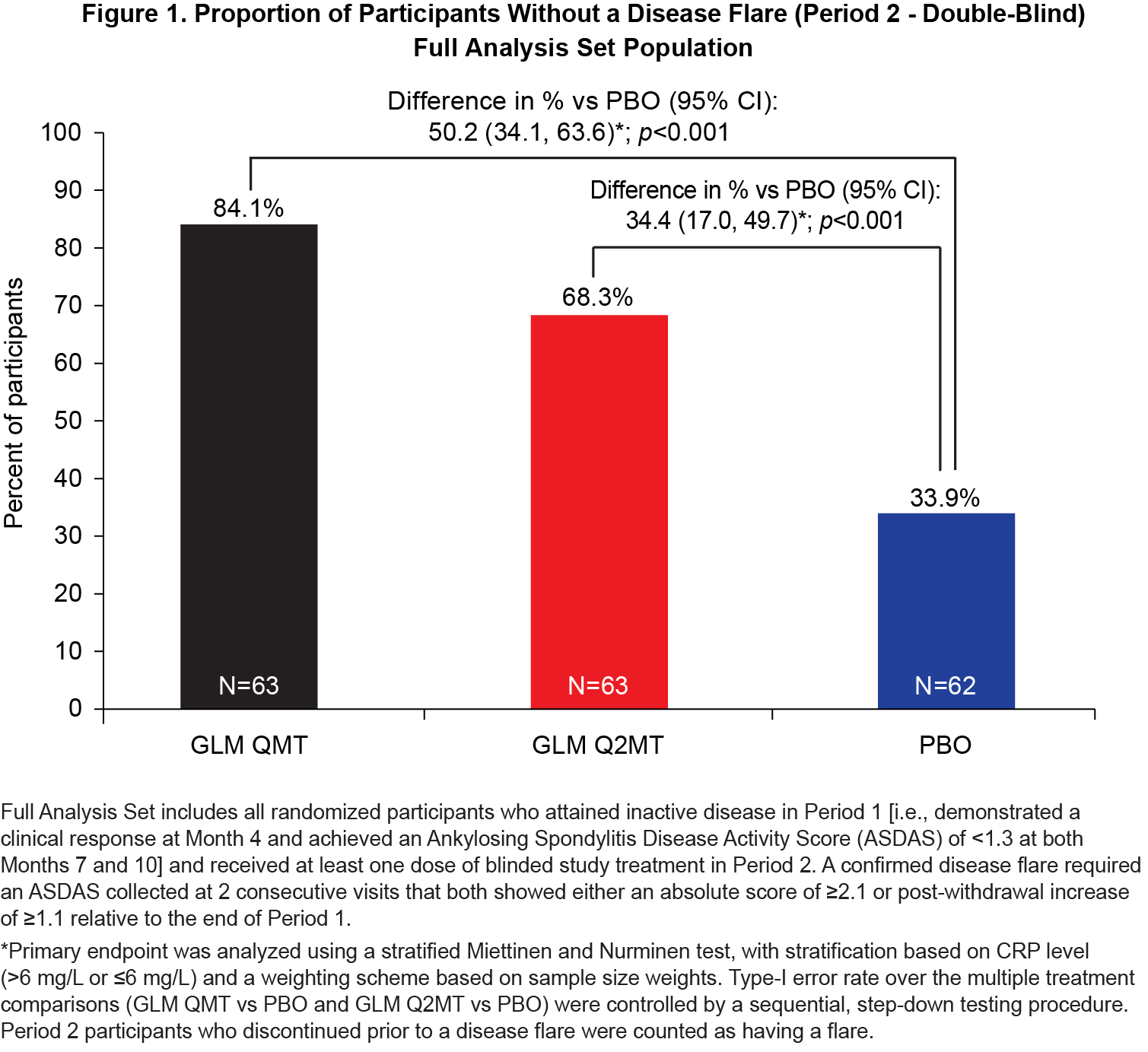 Figure 1
Figure 1
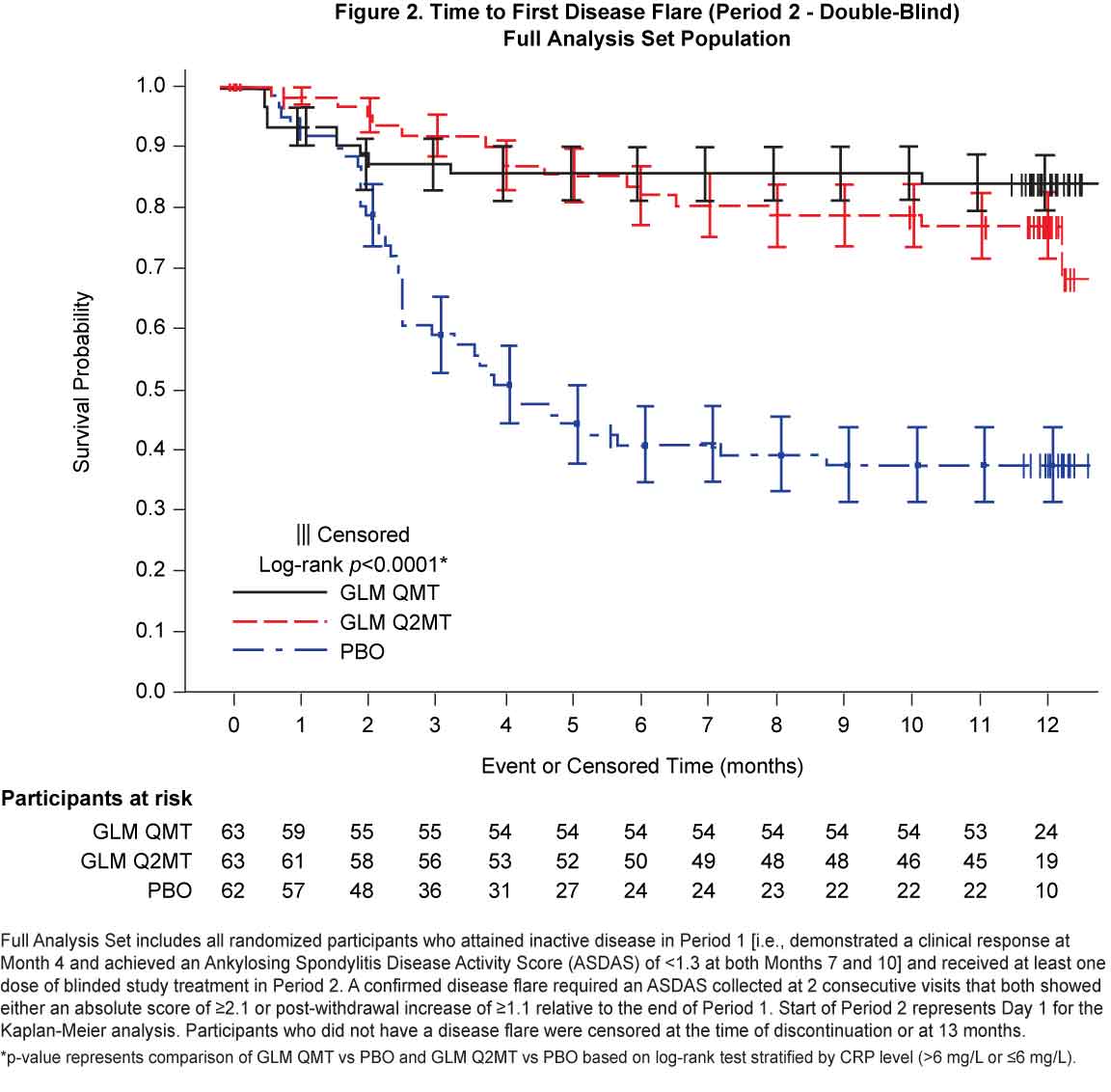 Figure 2
Figure 2
Disclosures: C. Weinstein, Merck/MSD; P. Sliwinska-Stanczyk, None; T. Hala, None; M. Stanislav, None; A. Tzontcheva, Merck/MSD; R. Yao, Merck/MSD; Y. Berd, Merck/MSD; S. Curtis, Merck/MSD; G. Philip, Merck/MSD.
Background/Purpose: Non-radiographic axial spondyloarthrits (nr-axSpA) is a rheumatic disease that is predominantly associated with chronic back pain and stiffness.1 Results from the Phase III GO-AHEAD trial demonstrate the efficacy and safety of golimumab (GLM) in the treatment of nr-axSpA;2,3 however, the efficacy and safety of GLM withdrawal has not been studied. This study evaluates the effect of GLM withdrawal on the incidence of a flare in disease activity and to characterize clinical response after GLM retreatment for a disease flare.
Methods: This Phase-IV parallel-group withdrawal study (GO-BACK) included adult participants, aged 18-45 years, with active nr-axSpA for ≤ 5 years, chronic back pain of ≥ 3 months, objective signs of inflammation, and intolerance or inadequate response to non-steroid anti-inflammatory drugs. In Period 1, participants received open-label (OL) subcutaneous GLM monthly for up to 10 months. In Period 2, participants who achieved inactive disease were randomized 1:1:1 to either receive monthly placebo (PBO, treatment withdrawal), or continued GLM treatment given monthly (GLM QMT, full-treatment) or every 2-months (GLM Q2MT, reduced-treatment). Participants who did not have a disease flare continued randomized treatment for ~12 months, whereas participants with a disease flare discontinued randomized treatment and resumed monthly dosing with OL GLM. All participants were followed for safety until ~3 months after their last dose. The primary endpoint was the proportion of participants without a disease flare on continued GLM treatment (GLM QMT or GLM Q2MT) versus GLM withdrawal (PBO). Among participants randomized to GLM QMT or PBO who experienced a disease flare, clinical response to monthly GLM retreatment was evaluated as a secondary endpoint.
Results: Of the 323 Period 1 participants, 188 achieved inactive disease and were eligible for treatment withdrawal. At baseline, Period 2 participants had a median disease symptom duration of 8 y, a median age of 32.0 y, 70.4% were male and all were White. Continued GLM treatment (QMT or Q2MT) was superior to treatment withdrawal (p< 0.001), with a greater proportion of GLM QMT participants having no disease flares compared to GLM Q2MT (50.1% vs 34.4%) [Figure 1]. Similarly, the time to first flare was significantly longer (log-rank p < 0.0001) for participants continuing GLM treatment compared with the PBO group [Figure 2]. Of the 53 participants who had a confirmed disease flare, 51 (96.2%) attained a clinical response to GLM within the first 3 months of retreatment. Adverse events (AEs) were consistent with the known safety profile for GLM. Throughout the study, there were no deaths or serious drug-related adverse events (AEs). In Period 2, the incidences of serious AEs (1.6%) and non-serious drug-related AEs (5.8%) were low, and both were comparable across the treatment groups.
Conclusion: Among participants with active nr-axSpA who attained inactive disease after 10 months of GLM treatment, continued GLM treatment provides superior protection against disease flares compared to GLM withdrawal. GLM was generally safe and well-tolerated, with a comparable incidence of AEs across all treatment groups.
 Figure 1
Figure 1 Figure 2
Figure 2Disclosures: C. Weinstein, Merck/MSD; P. Sliwinska-Stanczyk, None; T. Hala, None; M. Stanislav, None; A. Tzontcheva, Merck/MSD; R. Yao, Merck/MSD; Y. Berd, Merck/MSD; S. Curtis, Merck/MSD; G. Philip, Merck/MSD.

