Back
Abstract Session
Immunobiology
Session: Abstracts: Innate Immunity (0554–0557)
0555: Heterogeneity in Origin and Function of Distinct Human Synovial-tissue Dendritic Cells in Health, Active Rheumatoid Arthritis (RA) and RA in Disease Remission
Saturday, November 12, 2022
5:15 PM – 5:25 PM Eastern Time
Location: Room 114 Nutter Theatre
- DS
Domenico Somma, PhD
School of infection and immunity
Glasgow, United Kingdom
Presenting Author(s)
Aziza Elmesmari1, Lucy MacDonald2, Jack Frew2, Domenico Somma2, Clara Di Mario3, Audrey Paoletti4, Diane Vaughan5, Barbara Tolusso6, Simone Perniola6, Marco Gessi7, Leandro Lemgruber5, Maria Rita Gigante8, Luca P Petricca9, Laura Bui7, Dario Bruno3, charles McSharry5, John D Isaacs10, Iain B McInnes11, Simon Milling5, Elisa Gremese3, Thomas D Otto2, Kenneth Baker12, Stefano Alivernini6 and Mariola Kurowska-Stolarska1, 1Research into Inflammatory Arthritis Centre Versus Arthritis (RACE), University of Glasgow, Glasgow, Scotland, United Kingdom, 2Research into Inflammatory Arthritis Centre Versus Arthritis (RACE), University of Glasgow, Glasgow, Scotland, United Kingdom, 3Immunology Research Core Facility, Gemelli Science and Technology Park, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy, 4University of Glasgow, Glasgow, Scotland, United Kingdom, 5Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow, Scotland, United Kingdom, 6Immunology Research Core Facility, Gemelli Science and Technology Park, Fondazione Policlinico Universitario A. Gemelli IRCCS,, Rome, Italy, 7Institute of Pathology, Fondazione Policlinico Universitario A. Gemelli IRCCS,, Rome, Italy, 8Division of Rheumatology, Fondazione Policlinico Universitario A. Gemelli IRCCS,, Rome, United Kingdom, 9Division of Rheumatology, Fondazione Policlinico Universitario A. Gemelli IRCCS,, Rome, Italy, 10Institute for Translational and Clinical Research, Newcastle University and Musculoskeletal Unit, Newcastle upon Tyne Hospitals, Newcastle upon Tyne, United Kingdom, 11Institute of Infection, Immunity and Inflammation, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, Scotland, United Kingdom, 12Institute for Translational and Clinical Research, Newcastle University and Musculoskeletal Unit, Newcastle upon Tyne Hospitals, Newcastle, United Kingdom
Background/Purpose: Current treatments for RA do not restore the immune tolerance characteristic of health. Dendritic cells (DC) are one of the cell types that can reset adaptive immunity. Characterisation of synovial tissue (ST)-DCs in RA patients and heathy donors may reveal pathways that enable transition to cure.
Methods: ST-DCs from biopsies of remission RA, active RA and healthy synovium (SYNGem biobank1) were compared by scRNAseq, flow cytometry and synovial tissue imaging. Blood (PB) ST-DC precursors in remission (BioRRA biobank2) were evaluated by scRNAseq. Functions of sorted ST-DC clusters were tested by co-culture with autologous T-cells and in animal models.
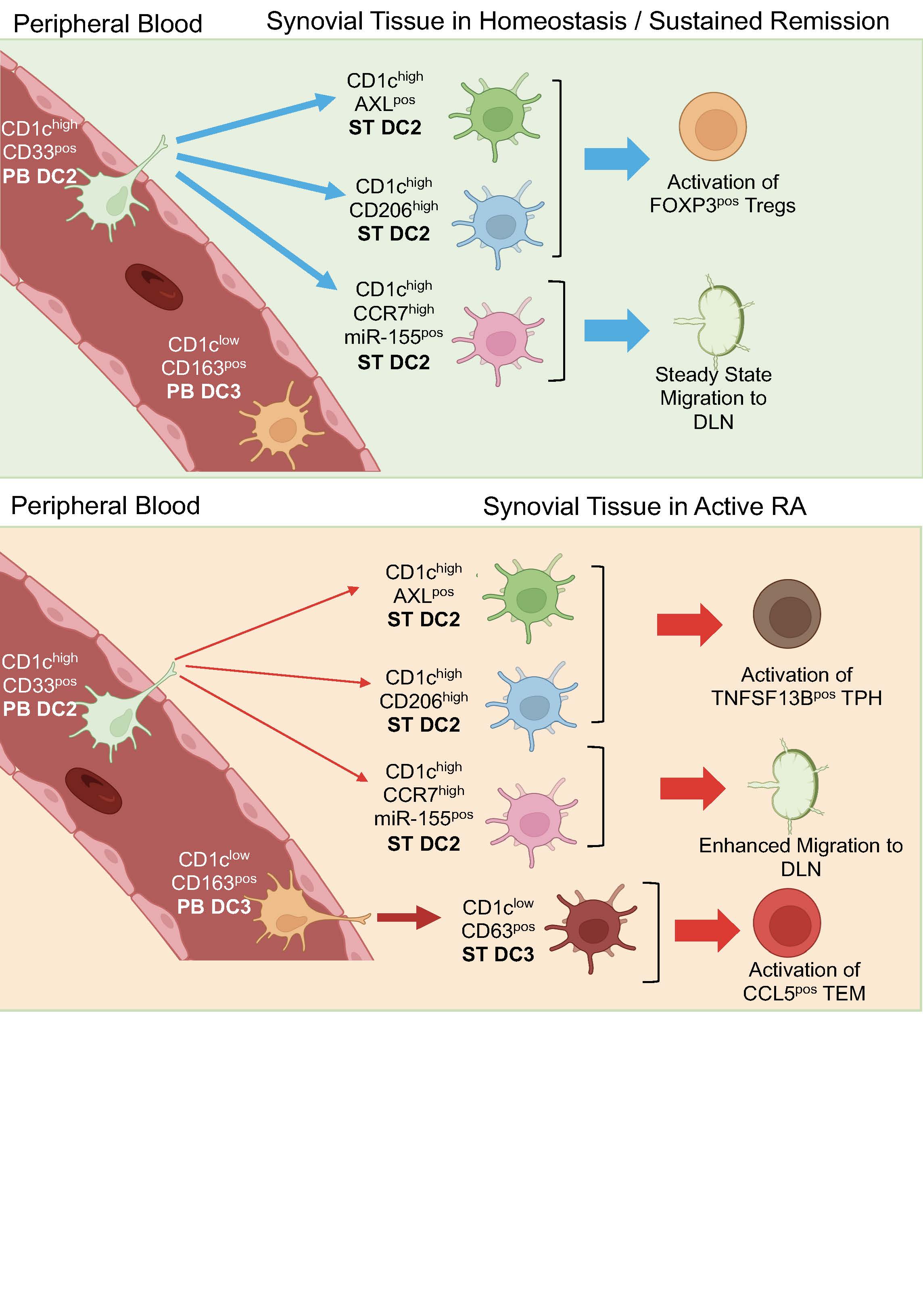
Results: The most abundant DC population in all human synovial tissues are CD1cpos and scRNAseq uncovered that these are highly heterogenous, revealing 5 distinct clusters. They differ in transcriptomics, functions, distribution, location, and ontogeny between joint conditions. Trajectory analysis identified that the 3 ST-CD1chigh clusters: AXLpos, CD206pos and CCR7pos differentiate from blood CD1chigh DC2 precursors, whereas the ST-CD1clowCD63pos cluster differentiated from blood DC3 (CD1clowCD163pos) precursors. The CD1chighAXLpos and CD1chighCD206pos are the most abundant clusters in healthy synovial tissues, and are located in the superficial sublining layer. The active RA synovium is characterised by emergence in the sublining layer of DC3-precursor derived CD1clowCD63pos DCs. Their number decreases significantly in remission, which is characterised by a dominant CD1chighCD206pos and absence of CD1chighAXLpos clusters typical of the healthy synovium. Some remission RA patients however retained a substantial CD1clowCD63pos cluster characteristic of active RA suggesting this or their blood DC3 precursors may be predictive of flare. Consistent with that, in BioRRA remission cohort, the specific transcriptomic signature of PB DC3 precursors were predictive of disease flare. FACS-sorted ST-DC2/3 clusters in co-cultures with autologous CD4pos memory T-cells demonstrated their distinct functions within and between joint conditions. ST-DC2s from active RA supported proliferation/survival of T-peripheral helper cells (Tph) that drives autoantibody production, and ST-DC3s supported chemokine enriched CCL5pos T-effector memory cells. In contrast, ST-DC2s from remission supported FOXP3posTIGITpos regulatory T-cells, suggesting tolerogenic function. ST-DC3 clusters if present in remission activated Tph cells, suggesting an activatory function and a role in disease flare. Mouse lymph-duct canulation revealed that ST-CD1chighCCR7high cluster has a migratory phenotype, and their migratory function and activation is enhanced in active RA and regulated by miR-155.
Conclusion: Distinct synovial tissue DC CD1cpos clusters may determine healthy immune tolerance, autoimmunity and disease remission. Therapeutic targeting of the stimulatory/inflammatory functions of ST-DC3 (CD63pos) in remission, and/or re-instating the healthy ST-DC2: CD1chighAXLpos and CD1chighCD206pos clusters might provide pathways to transform remission into healthy immune-homeostasis.
Ref.1 Alivernini et al. 2020
Ref.2 Baker et al. 2019
Disclosures: A. Elmesmari, None; L. MacDonald, None; J. Frew, None; D. Somma, None; C. Di Mario, None; A. Paoletti, None; D. Vaughan, None; B. Tolusso, None; S. Perniola, None; M. Gessi, None; L. Lemgruber, None; M. Gigante, None; L. Petricca, None; L. Bui, None; D. Bruno, None; c. McSharry, None; J. Isaacs, AbbVie/Abbott, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Janssen, Eli Lilly, Gilead, Pfizer, Roche; I. McInnes, Bristol-Myers Squibb (BMS), Janssen, Novartis, UCB, Pfizer, AbbVie, Celgene, AstraZeneca, Boehringer Ingelheim, EveloBio, LEO, Lilly; S. Milling, None; E. Gremese, None; T. Otto, None; K. Baker, None; S. Alivernini, None; M. Kurowska-Stolarska, None.
Background/Purpose: Current treatments for RA do not restore the immune tolerance characteristic of health. Dendritic cells (DC) are one of the cell types that can reset adaptive immunity. Characterisation of synovial tissue (ST)-DCs in RA patients and heathy donors may reveal pathways that enable transition to cure.
Methods: ST-DCs from biopsies of remission RA, active RA and healthy synovium (SYNGem biobank1) were compared by scRNAseq, flow cytometry and synovial tissue imaging. Blood (PB) ST-DC precursors in remission (BioRRA biobank2) were evaluated by scRNAseq. Functions of sorted ST-DC clusters were tested by co-culture with autologous T-cells and in animal models.
Results: The most abundant DC population in all human synovial tissues are CD1cpos and scRNAseq uncovered that these are highly heterogenous, revealing 5 distinct clusters. They differ in transcriptomics, functions, distribution, location, and ontogeny between joint conditions. Trajectory analysis identified that the 3 ST-CD1chigh clusters: AXLpos, CD206pos and CCR7pos differentiate from blood CD1chigh DC2 precursors, whereas the ST-CD1clowCD63pos cluster differentiated from blood DC3 (CD1clowCD163pos) precursors. The CD1chighAXLpos and CD1chighCD206pos are the most abundant clusters in healthy synovial tissues, and are located in the superficial sublining layer. The active RA synovium is characterised by emergence in the sublining layer of DC3-precursor derived CD1clowCD63pos DCs. Their number decreases significantly in remission, which is characterised by a dominant CD1chighCD206pos and absence of CD1chighAXLpos clusters typical of the healthy synovium. Some remission RA patients however retained a substantial CD1clowCD63pos cluster characteristic of active RA suggesting this or their blood DC3 precursors may be predictive of flare. Consistent with that, in BioRRA remission cohort, the specific transcriptomic signature of PB DC3 precursors were predictive of disease flare. FACS-sorted ST-DC2/3 clusters in co-cultures with autologous CD4pos memory T-cells demonstrated their distinct functions within and between joint conditions. ST-DC2s from active RA supported proliferation/survival of T-peripheral helper cells (Tph) that drives autoantibody production, and ST-DC3s supported chemokine enriched CCL5pos T-effector memory cells. In contrast, ST-DC2s from remission supported FOXP3posTIGITpos regulatory T-cells, suggesting tolerogenic function. ST-DC3 clusters if present in remission activated Tph cells, suggesting an activatory function and a role in disease flare. Mouse lymph-duct canulation revealed that ST-CD1chighCCR7high cluster has a migratory phenotype, and their migratory function and activation is enhanced in active RA and regulated by miR-155.
Conclusion: Distinct synovial tissue DC CD1cpos clusters may determine healthy immune tolerance, autoimmunity and disease remission. Therapeutic targeting of the stimulatory/inflammatory functions of ST-DC3 (CD63pos) in remission, and/or re-instating the healthy ST-DC2: CD1chighAXLpos and CD1chighCD206pos clusters might provide pathways to transform remission into healthy immune-homeostasis.
Ref.1 Alivernini et al. 2020
Ref.2 Baker et al. 2019

Disclosures: A. Elmesmari, None; L. MacDonald, None; J. Frew, None; D. Somma, None; C. Di Mario, None; A. Paoletti, None; D. Vaughan, None; B. Tolusso, None; S. Perniola, None; M. Gessi, None; L. Lemgruber, None; M. Gigante, None; L. Petricca, None; L. Bui, None; D. Bruno, None; c. McSharry, None; J. Isaacs, AbbVie/Abbott, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Janssen, Eli Lilly, Gilead, Pfizer, Roche; I. McInnes, Bristol-Myers Squibb (BMS), Janssen, Novartis, UCB, Pfizer, AbbVie, Celgene, AstraZeneca, Boehringer Ingelheim, EveloBio, LEO, Lilly; S. Milling, None; E. Gremese, None; T. Otto, None; K. Baker, None; S. Alivernini, None; M. Kurowska-Stolarska, None.

