Back
Poster Session D
Myopathic rheumatic diseases (polymyositis, dermatomyositis, inclusion body myositis)
Session: (1856–1887) Muscle Biology, Myositis and Myopathies Poster II
1860: Lenabasum, a Cannabinoid Type 2 Receptor Agonist, Activates Diverse Cell-specific Pathways in Whole Blood Leukocytes in Dermatomyositis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- NK
Nilesh Kodali, BS
New Jersey Medical School
Coppell, TX, United States
Abstract Poster Presenter(s)
Nilesh Kodali1, Thomas Vazquez2, DeAnna Diaz3, Josh Dan4, Grant Sprow5, Rohan Dhiman6, Mariko Ogawa-Momohara7, Muhammad Bashir6, Meena Sharma8 and Victoria Werth9, 1New Jersey Medical School, Coppell, TX, 2FIU Wertheim College of Medicine, Virginia Beach, VA, 3Philadelphia College of Medicine, Philadelphia, PA, 4Philadelphia VAMC, Philadelphia, PA, USA and Department of Dermatology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, 5Albert Einstein College of Medicine, Philadelphia, PA, 6University of Pennsylvania, Philadelphia, PA, 7Nagoya University Graduate School of Medicine, Nagoya, Japan, 8Philadelphia VAMC, Philadelphia, PA, USA and Department of Dermatology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, 9University of Pennsylvania and Corporal Michael J. Crescenz VA Medical Center, Philadelphia, PA
Background/Purpose: Dermatomyositis (DM) is a systemic autoimmune disease that affects the skin and muscle. Lenabasum, a cannabinoid type 2 receptor (CB2R) agonist, has been developed as a potential treatment to reduce inflammation in DM. Additional research has also shown that lenabasum also exerts its anti-inflammatory effects through the binding of peroxisome proliferator-activated receptor gamma (PPARγ).
Methods: We investigated the downstream lenabasum-specfic pathway preference through CB2R or PPARγ in various leukocyte cell lines. We used IFNβ, a major driver of DM, as a marker for inflammation and various combinations of CB2R and PPARγ inhibitors on DM whole blood leukocytes. Prior research has shown that activation of CB2R allows for the recruitment of cyclooxygenase 2 (COX2) and lipoxygenase enzymes (15LOX). These enzymes were utilized as biomarkers for CB2R pathway activation. Flow cytometry was used to quantify differences in IFNβ levels with various CB2R/PPARγ inhibitions in the presence of lenabasum (15μmol).
Results: We found that lenabasum acts through the CB2R in CD4T+ cells due to a significant reduction (Median Frequency of Parent [MFOP] from 12.6% to 3.46%; p< 0.05) in IFNβ upon treatment with lenabasum but a slight nonsignificant decrease (MFOP from 7.56% to 4.46%; p >0.05) when CB2 receptors were inhibited (Figure 1a). Downstream lenabasum-specific CB2R pathway activation was confirmed in these cells with a significant increase in COX2 (MFOP from 3.16% to 18.715%; p< 0.05) and 15LOX (MFOP from 5.01% to 12.32%; p< 0.05) upon treatment with lenabasum in DM responders versus a non-significant increase in these enzymes (MFOP from 3.63% to 9.215%; p >0.05) in DM non-responders (Figure 2a). Plasmacytoid dendritic cells (pDCs) favor a PPARγ-dominant pathway due to the significant reduction in IFNβ levels (MFOP from 9.964% to 2.86%) when treating with lenabasum with or without CB2R inhibition, but a non-significant decrease (MFOP from 55.21% to 42.246%) when inhibiting PPARγ (Figure 1b). Both COX2 and 15LOX levels had no significant increase when treating with lenabasum in DM responders, suggesting a non-CB2R pathway in pDCs (Figure 2c). The myeloid dendritic cells (mDCs) demonstrated an ability to activate both CB2R and PPARγ pathways. In these cell lines, lenabasum significantly reduced IFNβ levels when either CB2R (MFOP from 9.545% to 3.2215%) or PPARγ (MFOP from 46.105% to 21.775%) were inhibited independently. However, when both receptors were inhibited simultaneously, treatment with lenabasum showed a nonsignificant increase (MFOP from 28.665% to 40.91%) in IFNβ levels (Figure 1c). COX2 and 15LOX levels were also significantly increased in mDCs when treated with lenabasum in the DM responders versus the DM non-responders, suggesting a CB2R pathway activation (Figure 2b).
Conclusion: Altogether, these results show that lenabasum has a cell-specific pathway preference in the blood and the efficacy of the treatment could vary across patients due to potential baseline cell-to-cell differences in CB2R and PPARγ levels.
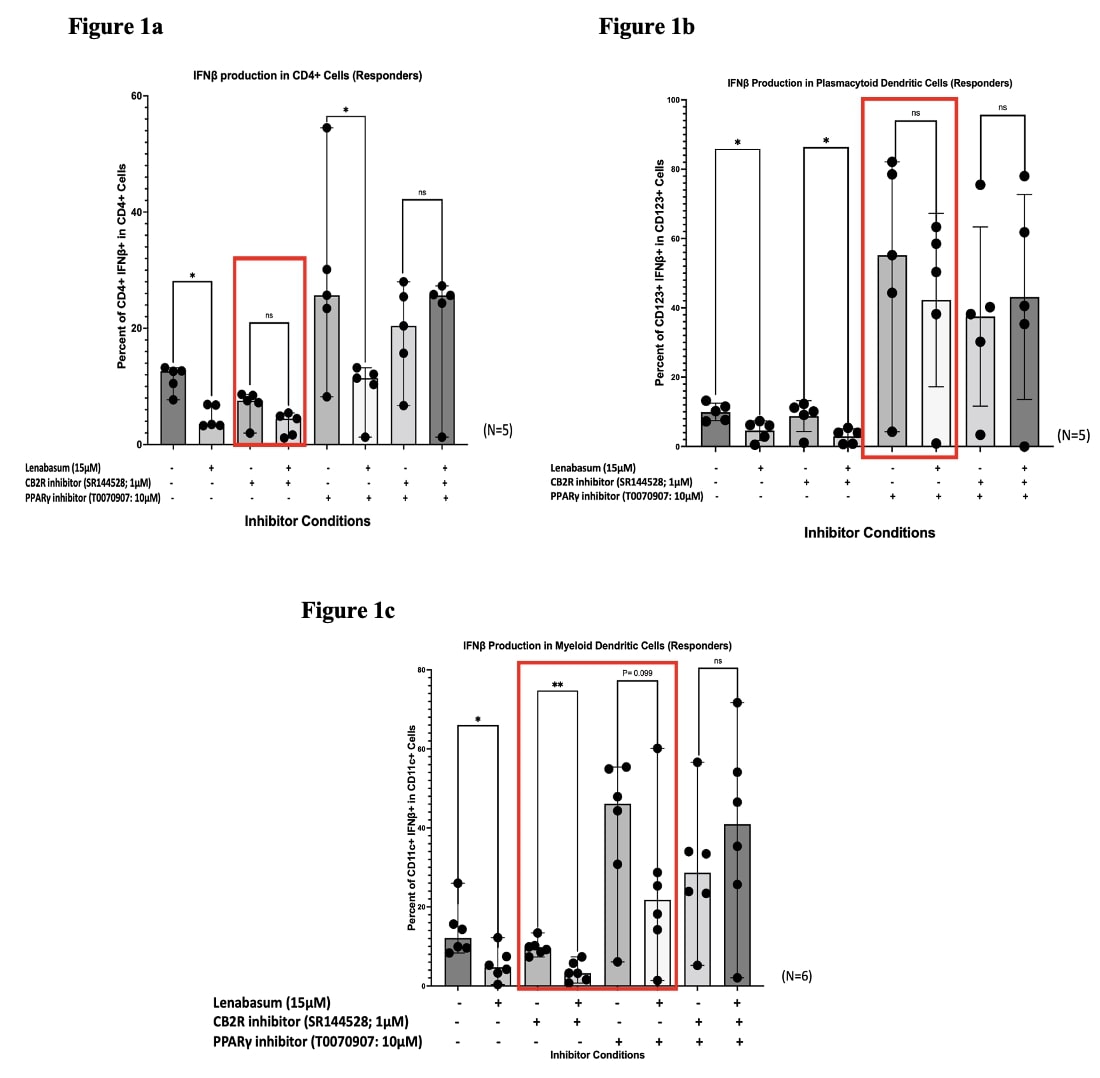 Figure 1. Flow Cytometry of IFNβ levels in DM whole blood leukocytes show a diverse cell-specific pathway activation upon treatment with lenabasum.
Figure 1. Flow Cytometry of IFNβ levels in DM whole blood leukocytes show a diverse cell-specific pathway activation upon treatment with lenabasum.
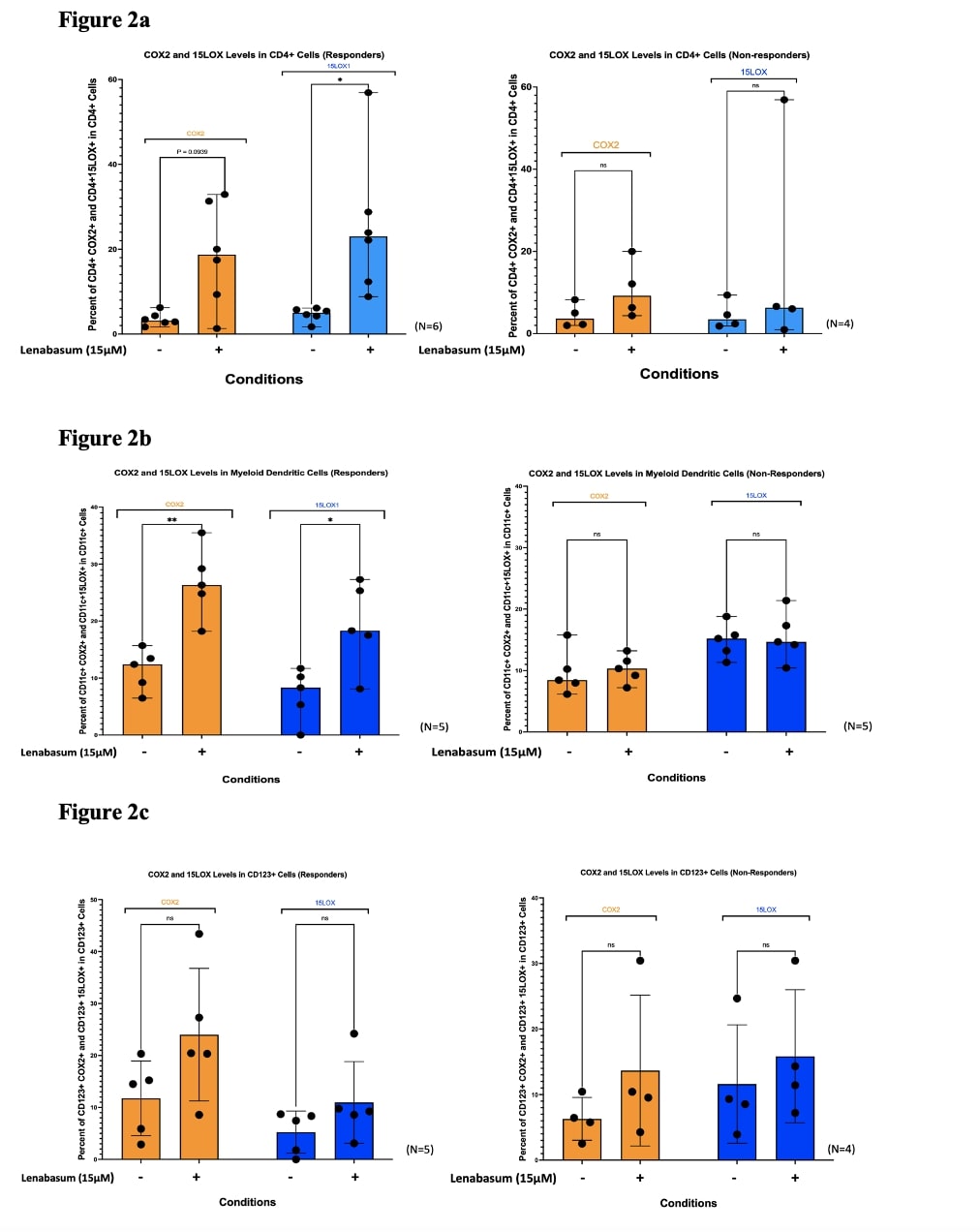 Figure 2. Flow Cytometry of COX2 and 15LOX1 levels in DM whole blood leukocytes across various cell lines in DM responders and non-responders to lenabasum. Increased enzyme levels in DM responders suggest CB2R pathway activation.
Figure 2. Flow Cytometry of COX2 and 15LOX1 levels in DM whole blood leukocytes across various cell lines in DM responders and non-responders to lenabasum. Increased enzyme levels in DM responders suggest CB2R pathway activation.
Disclosures: N. Kodali, None; T. Vazquez, None; D. Diaz, None; J. Dan, None; G. Sprow, None; R. Dhiman, None; M. Ogawa-Momohara, None; M. Bashir, None; M. Sharma, None; V. Werth, AbbVie/Abbott, Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Celgene, Eli Lilly, Genentech, GlaxoSmithKline (GSK), Janssen, Merck/MSD, Gilead, Novartis, Pfizer, Rome Pharmaceuticals, Horizon Therapeutics, Regeneron, argenx, CSL Behring, AnaptysBio, Biogen, Corbus, EMD Serono, Galderma, Nektar, Octapharma, Principia, Resolve, Sanofi, Syntimmune, Viela.
Background/Purpose: Dermatomyositis (DM) is a systemic autoimmune disease that affects the skin and muscle. Lenabasum, a cannabinoid type 2 receptor (CB2R) agonist, has been developed as a potential treatment to reduce inflammation in DM. Additional research has also shown that lenabasum also exerts its anti-inflammatory effects through the binding of peroxisome proliferator-activated receptor gamma (PPARγ).
Methods: We investigated the downstream lenabasum-specfic pathway preference through CB2R or PPARγ in various leukocyte cell lines. We used IFNβ, a major driver of DM, as a marker for inflammation and various combinations of CB2R and PPARγ inhibitors on DM whole blood leukocytes. Prior research has shown that activation of CB2R allows for the recruitment of cyclooxygenase 2 (COX2) and lipoxygenase enzymes (15LOX). These enzymes were utilized as biomarkers for CB2R pathway activation. Flow cytometry was used to quantify differences in IFNβ levels with various CB2R/PPARγ inhibitions in the presence of lenabasum (15μmol).
Results: We found that lenabasum acts through the CB2R in CD4T+ cells due to a significant reduction (Median Frequency of Parent [MFOP] from 12.6% to 3.46%; p< 0.05) in IFNβ upon treatment with lenabasum but a slight nonsignificant decrease (MFOP from 7.56% to 4.46%; p >0.05) when CB2 receptors were inhibited (Figure 1a). Downstream lenabasum-specific CB2R pathway activation was confirmed in these cells with a significant increase in COX2 (MFOP from 3.16% to 18.715%; p< 0.05) and 15LOX (MFOP from 5.01% to 12.32%; p< 0.05) upon treatment with lenabasum in DM responders versus a non-significant increase in these enzymes (MFOP from 3.63% to 9.215%; p >0.05) in DM non-responders (Figure 2a). Plasmacytoid dendritic cells (pDCs) favor a PPARγ-dominant pathway due to the significant reduction in IFNβ levels (MFOP from 9.964% to 2.86%) when treating with lenabasum with or without CB2R inhibition, but a non-significant decrease (MFOP from 55.21% to 42.246%) when inhibiting PPARγ (Figure 1b). Both COX2 and 15LOX levels had no significant increase when treating with lenabasum in DM responders, suggesting a non-CB2R pathway in pDCs (Figure 2c). The myeloid dendritic cells (mDCs) demonstrated an ability to activate both CB2R and PPARγ pathways. In these cell lines, lenabasum significantly reduced IFNβ levels when either CB2R (MFOP from 9.545% to 3.2215%) or PPARγ (MFOP from 46.105% to 21.775%) were inhibited independently. However, when both receptors were inhibited simultaneously, treatment with lenabasum showed a nonsignificant increase (MFOP from 28.665% to 40.91%) in IFNβ levels (Figure 1c). COX2 and 15LOX levels were also significantly increased in mDCs when treated with lenabasum in the DM responders versus the DM non-responders, suggesting a CB2R pathway activation (Figure 2b).
Conclusion: Altogether, these results show that lenabasum has a cell-specific pathway preference in the blood and the efficacy of the treatment could vary across patients due to potential baseline cell-to-cell differences in CB2R and PPARγ levels.
 Figure 1. Flow Cytometry of IFNβ levels in DM whole blood leukocytes show a diverse cell-specific pathway activation upon treatment with lenabasum.
Figure 1. Flow Cytometry of IFNβ levels in DM whole blood leukocytes show a diverse cell-specific pathway activation upon treatment with lenabasum. Figure 2. Flow Cytometry of COX2 and 15LOX1 levels in DM whole blood leukocytes across various cell lines in DM responders and non-responders to lenabasum. Increased enzyme levels in DM responders suggest CB2R pathway activation.
Figure 2. Flow Cytometry of COX2 and 15LOX1 levels in DM whole blood leukocytes across various cell lines in DM responders and non-responders to lenabasum. Increased enzyme levels in DM responders suggest CB2R pathway activation. Disclosures: N. Kodali, None; T. Vazquez, None; D. Diaz, None; J. Dan, None; G. Sprow, None; R. Dhiman, None; M. Ogawa-Momohara, None; M. Bashir, None; M. Sharma, None; V. Werth, AbbVie/Abbott, Amgen, AstraZeneca, Bristol Myers Squibb (BMS), Celgene, Eli Lilly, Genentech, GlaxoSmithKline (GSK), Janssen, Merck/MSD, Gilead, Novartis, Pfizer, Rome Pharmaceuticals, Horizon Therapeutics, Regeneron, argenx, CSL Behring, AnaptysBio, Biogen, Corbus, EMD Serono, Galderma, Nektar, Octapharma, Principia, Resolve, Sanofi, Syntimmune, Viela.

