Back
Abstract Session
Immunobiology
Session: Abstracts: Cytokines and Cell Trafficking (0558–0561)
0558: Molecular Interplay Between Mammalian Target of Rapamycin (mTOR) and the YAP Pathway Mediates Rheumatoid Arthritis Synovial Fibroblast Activation
Sunday, November 13, 2022
8:00 AM – 8:10 AM Eastern Time
Location: Room 103
- MC
Mary Canavan, PhD
Trinity College
Dublin, Dublin, Ireland
Presenting Author(s)
Brianne Barker1, Megan Hanlon2, Siobhan Wade1, Douglas Veale3, Ursula Fearon1 and Mary Canavan4, 1Trinity College Dublin, Dublin, Ireland, 2Molecular Rheumatology, Dublin, Ireland, 3St. Vincent's University Hospital, Blackrock, Dublin, Ireland, 4Trinity College, Dublin, Ireland
Background/Purpose: mTOR is a metabolic master regulator of both innate and adaptive immunity, however its exact role in synovial fibroblasts is unknown. RA synovial fibroblasts (RASFC) are known to mediate cartilage destruction and bone degradation. We therefore aimed to explore the molecular role of mTOR in RASFC activation, metabolism and invasion. Furthermore, we also aimed to explore the role of the Hippo-YAP pathway in RASFC and determine if molecular crosstalk between the pathways meditates RASFC activation.
Methods: Healthy control (HC) and RA synovial fibroblasts were stained for mTOR and analysed by Flow Cytometry. RNA-Seq analysis was performed on HC, Individuals At Risk (IAR) and RA synovial tissue biopsies. Data was analysed to detect differential gene and pathway expression and was deposited in GEO database (GSE89408). RASFC were cultured in the presence of TNFα (1ng/ml) alone or in combination with the mTOR inhibitor Rapamycin (100nM) or YAP inhibitor Verteporfin (50nM). ELISA, RT-PCR and Flow Cytometry were used to measure cytokine/chemokine production and expression of adhesion markers. Cellular invasion was assessed using Matrigel invasion assays while cellular bioenergetics was assessed using Seahorse XFe-Technology.
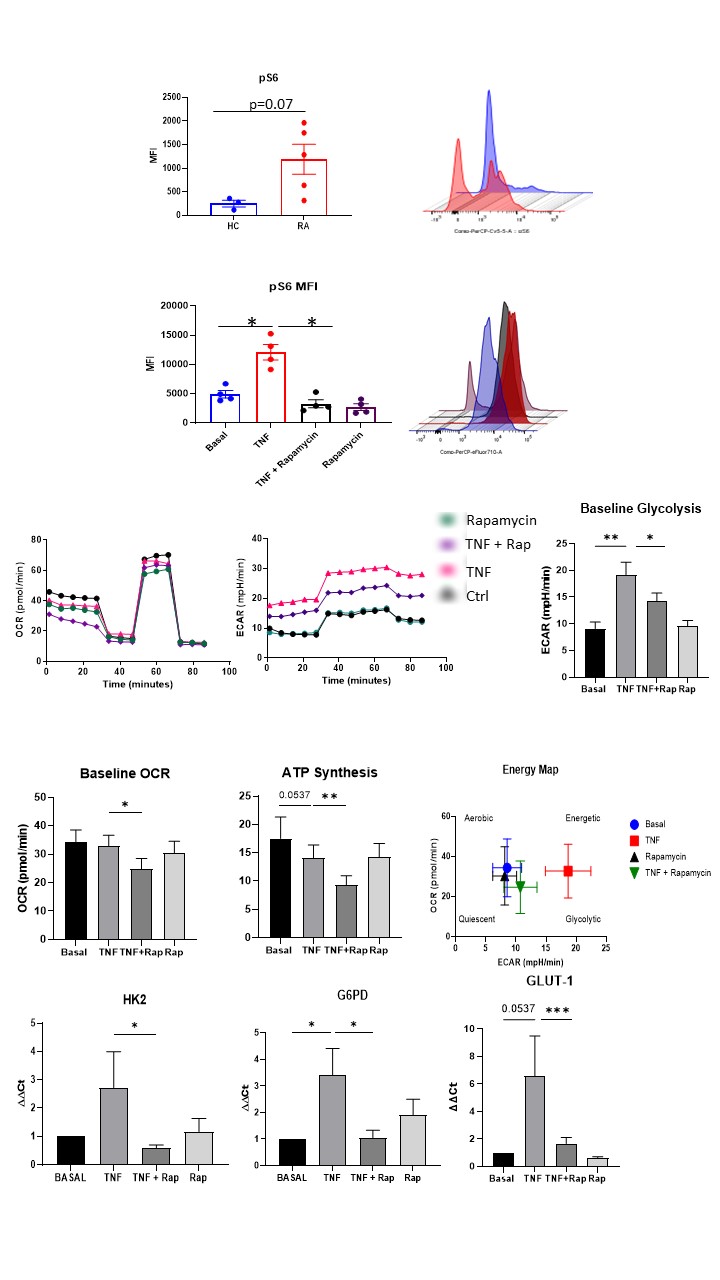
Results: RNASeq analysis demonstrated differential expression of genes related to the mTOR pathway in HC v RA synovial biopsies. Specifically mTOR was significantly increased in RA fibroblasts compared to HC (p< 0.05). Rapamycin inhibits TNFα mediated mTOR activatiotn (pS6 induction (p< 0.05) while also inhibiting TNFα induced ECAR (measure of glycolysis), OCR (measure of OXPHOS) and ATP synthesis in addition to several glycolytic genes (HK2, G6PD, p< 0.05). Rapamycin significantly decreases cytokines/chemokines IL-6, MCP-1, RANTES, adhesion molecules ICAM-1 and MMPs (MMP-1; all p< 0.05) while also inhibiting TNFα induced migration and invasion (both p< 0.05). Mechanistically, TNFα induces YAP expression which is abrogated in the presence of Rapamycin. TNFα induced MMP-1/MMP-3 expression and RASFC invasion is abrogated in the presence of YAP inhibition. Finally, RNASeq analysis revealed increased expression of mTOR pathway signature in IAR suggesting its potential role in early disease initiation.
Conclusion: Molecular crosstalk via the mTOR and YAP pathways mediate RASFC proinflammatory mechanisms.
 mTOR pathway drives metabolic reprogramming in RASFC
mTOR pathway drives metabolic reprogramming in RASFC
Disclosures: B. Barker, None; M. Hanlon, None; S. Wade, None; D. Veale, AbbVie/Abbott, Pfizer, Eli Lilly, Novartis, Gilead; U. Fearon, AbbVie/Abbott, Pfizer, Janssen, Eli Lilly, GlaxoSmithKlein(GSK); M. Canavan, None.
Background/Purpose: mTOR is a metabolic master regulator of both innate and adaptive immunity, however its exact role in synovial fibroblasts is unknown. RA synovial fibroblasts (RASFC) are known to mediate cartilage destruction and bone degradation. We therefore aimed to explore the molecular role of mTOR in RASFC activation, metabolism and invasion. Furthermore, we also aimed to explore the role of the Hippo-YAP pathway in RASFC and determine if molecular crosstalk between the pathways meditates RASFC activation.
Methods: Healthy control (HC) and RA synovial fibroblasts were stained for mTOR and analysed by Flow Cytometry. RNA-Seq analysis was performed on HC, Individuals At Risk (IAR) and RA synovial tissue biopsies. Data was analysed to detect differential gene and pathway expression and was deposited in GEO database (GSE89408). RASFC were cultured in the presence of TNFα (1ng/ml) alone or in combination with the mTOR inhibitor Rapamycin (100nM) or YAP inhibitor Verteporfin (50nM). ELISA, RT-PCR and Flow Cytometry were used to measure cytokine/chemokine production and expression of adhesion markers. Cellular invasion was assessed using Matrigel invasion assays while cellular bioenergetics was assessed using Seahorse XFe-Technology.
Results: RNASeq analysis demonstrated differential expression of genes related to the mTOR pathway in HC v RA synovial biopsies. Specifically mTOR was significantly increased in RA fibroblasts compared to HC (p< 0.05). Rapamycin inhibits TNFα mediated mTOR activatiotn (pS6 induction (p< 0.05) while also inhibiting TNFα induced ECAR (measure of glycolysis), OCR (measure of OXPHOS) and ATP synthesis in addition to several glycolytic genes (HK2, G6PD, p< 0.05). Rapamycin significantly decreases cytokines/chemokines IL-6, MCP-1, RANTES, adhesion molecules ICAM-1 and MMPs (MMP-1; all p< 0.05) while also inhibiting TNFα induced migration and invasion (both p< 0.05). Mechanistically, TNFα induces YAP expression which is abrogated in the presence of Rapamycin. TNFα induced MMP-1/MMP-3 expression and RASFC invasion is abrogated in the presence of YAP inhibition. Finally, RNASeq analysis revealed increased expression of mTOR pathway signature in IAR suggesting its potential role in early disease initiation.
Conclusion: Molecular crosstalk via the mTOR and YAP pathways mediate RASFC proinflammatory mechanisms.
 mTOR pathway drives metabolic reprogramming in RASFC
mTOR pathway drives metabolic reprogramming in RASFCDisclosures: B. Barker, None; M. Hanlon, None; S. Wade, None; D. Veale, AbbVie/Abbott, Pfizer, Eli Lilly, Novartis, Gilead; U. Fearon, AbbVie/Abbott, Pfizer, Janssen, Eli Lilly, GlaxoSmithKlein(GSK); M. Canavan, None.

