Back
Poster Session D
Epidemiology, health policy and outcomes
Session: (1750–1786) Epidemiology and Public Health Poster III
1785: PROs and Sociodemographic Factors in Year Prior to COVID Predict Trajectories of Depressive Symptoms in Adults with RA in First 2 Years of Pandemic: Data from the Canadian Early Arthritis Cohort
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- SB
Susan Bartlett, PhD
Professor of Medicine
McGill University
Montreal, QC, Canada
Abstract Poster Presenter(s)
Susan Bartlett1, orit schieir2, Marie-France Valois2, Janet Pope3, Louis Bessette4, Gilles Boire5, Carol Hitchon6, Edward Keystone7, Carter Thorne8, Diane Tin9, Glen Hazlewood10 and Vivian Bykerk11, 1McGill University, Montreal, QC, Canada, 2McGill University, Montréal, QC, Canada, 3University of Western Ontario, London, ON, Canada, 4Centre de l'Ostoporose et de Rhumatologie de Québec, Québec, QC, Canada, 5Universite de Sherbrooke, Sherbrooke, QC, Canada, 6University of Manitoba, Winnipeg, MB, Canada, 7Keystone Consulting Enterprises Inc., Toronto, ON, Canada, 8Southlake Regional Health Centre, Newmarket, ON, Canada, 9The Arthritis Program Research Group, Newmarket, ON, Canada, 10University of Calgary, Calgary, AB, Canada, 11Hospital for Special Surgery, New York, NY
Background/Purpose: Growing evidence points to considerable mental health impacts of the prolonged COVID-19 pandemic, though data from longitudinal studies in rheumatic diseases are sparse. We explored distinct trajectories of depressive symptoms in the year prior to and throughout the first 2-years of the COVID-19 pandemic in adults with RA .
Methods: The Canadian Early Arthritis Cohort (CATCH) is a prospective multi-center study of adults with early RA (symptoms < 1 year; 81% met 2010 ACR/EULAR criteria at enrolment) who receive care from rheumatologists across Canada. Prior to the pandemic, participants completed patient-reported assessments of symptoms and function (i.e., PROMIS-29 and patient global), and rheumatologists conducted RA assessments during scheduled in-person study visits. After March 2020, ongoing collection of key outcomes continued at in-person and remote visits.
We used group-based trajectory modeling to identify latent groups of participants with at least mild depression (PROMIS 4a depression score ≥55) in participants with ≥1 visits in the year prior to the pandemic (3/19-2/20) and ≥1 visits during pandemic (3/20-1/22) and identified prepandemic individual and clinical characteristics and PROs associated with each trajectory.
Results: The analytic sample included 989 participants with a mean (SD) age of 60 (14) and disease duration of 6 (4) years. 73% were women, 84% white, 60% had completed some post-secondary education, and 77% were in CDAI REM/LDA at visit closest to the start of pandemic.
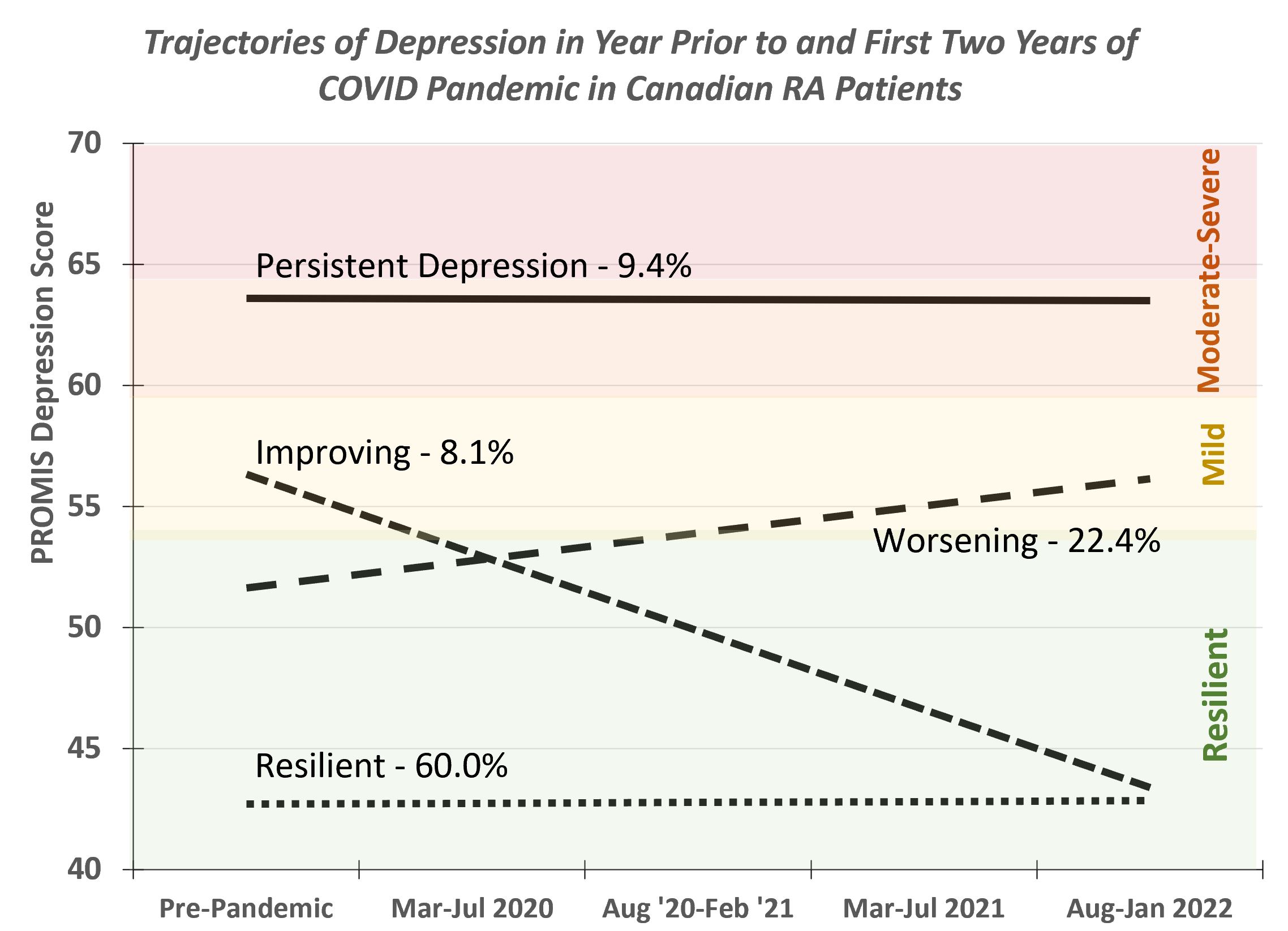
The best model included 4 groups (posterior probabilities ≥0.80 for each group): 1) Resilient (none-minimal depression throughout: N=594; 60%); Worsening (none/minimal to mild: N=222;22%); Improving (mild-resilient: N=80;8%); and Persistent (moderate-severe throughout: N=93;9%)(see figure). As compared with the Resilient group, those with Worsening Depression were more likely to be female, obese, have a higher pre-pandemic CDAI, MD and patient global, and report worse pain, disability, anxiety, depression, fatigue, sleep disturbance, and lower participation (Table).
Conclusion: Although 60% of Canadian RA patients had consistently good mental health during the first 2 years of the COVID-19 pandemic, more than 1 in 5 reported deteriorating mood suggesting a cumulative impact over time; 9% had persistent depression and 8% improving mood. The proportion of adults with RA with at least mild symptoms of depression may be more than twice that reported for the general Canadian population. As compared with those with good mental health throughout, participants with worsening depressive symptoms during the pandemic were more likely to be female, obese, have higher pre-pandemic disease activity, symptoms, disability, and higher impairments in participation. Given the impact of depression on quality of life, inflammation, and disease management, vulnerable groups may benefit from more frequent evaluation and additional support from rheumatology providers.
.jpg)
Disclosures: S. Bartlett, Pfizer, Novartis, Merck/MSD, Janssen, AbbVie/Abbott, Organon; o. schieir, None; M. Valois, None; J. Pope, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Eli Lilly, merk, Roche, Seattle Genetics, UCB, Actelion, Amgen, Bayer, Eicos Sciences, Emerald, Gilead, Janssen, Novartis, Pfizer, Sandoz, Sanofi, Boehringer Ingelheim; L. Bessette, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi-Genzyme, UCB, Gilead, Merck/MSD, Organon, Roche; G. Boire, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Orimed Pharma, Pfizer, Samsung Bioepis, Teva, Viatris, Amgen, Celgene; C. Hitchon, Pfizer Canada, Astra-Zeneca Canada, Pfizer; E. Keystone, AbbVie/Abbott, Amgen, Gilead, Eli Lilly, Merck/MSD, Pfizer, Sanofi, Bristol-Myers Squibb(BMS), Celltrion, Myriad Autoimmune, F. Hoffmann-La Roche Inc, Genentech, Janssen, Sandoz, Samsung Bioepsis, AstraZeneca, Samsung Bioepsis; C. Thorne, AbbVie/Abbott, Amgen, Celgene, CaREBiodam, Centocor, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Medexus/Medac, Merck; D. Tin, None; G. Hazlewood, None; V. Bykerk, Amgen, Bristol-Myers Squibb(BMS), Genzyme, Brainstorm, Gilead, Regeneron, UCB, Pfizer, Sanofi, Aventis.
Background/Purpose: Growing evidence points to considerable mental health impacts of the prolonged COVID-19 pandemic, though data from longitudinal studies in rheumatic diseases are sparse. We explored distinct trajectories of depressive symptoms in the year prior to and throughout the first 2-years of the COVID-19 pandemic in adults with RA .
Methods: The Canadian Early Arthritis Cohort (CATCH) is a prospective multi-center study of adults with early RA (symptoms < 1 year; 81% met 2010 ACR/EULAR criteria at enrolment) who receive care from rheumatologists across Canada. Prior to the pandemic, participants completed patient-reported assessments of symptoms and function (i.e., PROMIS-29 and patient global), and rheumatologists conducted RA assessments during scheduled in-person study visits. After March 2020, ongoing collection of key outcomes continued at in-person and remote visits.
We used group-based trajectory modeling to identify latent groups of participants with at least mild depression (PROMIS 4a depression score ≥55) in participants with ≥1 visits in the year prior to the pandemic (3/19-2/20) and ≥1 visits during pandemic (3/20-1/22) and identified prepandemic individual and clinical characteristics and PROs associated with each trajectory.
Results: The analytic sample included 989 participants with a mean (SD) age of 60 (14) and disease duration of 6 (4) years. 73% were women, 84% white, 60% had completed some post-secondary education, and 77% were in CDAI REM/LDA at visit closest to the start of pandemic.
The best model included 4 groups (posterior probabilities ≥0.80 for each group): 1) Resilient (none-minimal depression throughout: N=594; 60%); Worsening (none/minimal to mild: N=222;22%); Improving (mild-resilient: N=80;8%); and Persistent (moderate-severe throughout: N=93;9%)(see figure). As compared with the Resilient group, those with Worsening Depression were more likely to be female, obese, have a higher pre-pandemic CDAI, MD and patient global, and report worse pain, disability, anxiety, depression, fatigue, sleep disturbance, and lower participation (Table).
Conclusion: Although 60% of Canadian RA patients had consistently good mental health during the first 2 years of the COVID-19 pandemic, more than 1 in 5 reported deteriorating mood suggesting a cumulative impact over time; 9% had persistent depression and 8% improving mood. The proportion of adults with RA with at least mild symptoms of depression may be more than twice that reported for the general Canadian population. As compared with those with good mental health throughout, participants with worsening depressive symptoms during the pandemic were more likely to be female, obese, have higher pre-pandemic disease activity, symptoms, disability, and higher impairments in participation. Given the impact of depression on quality of life, inflammation, and disease management, vulnerable groups may benefit from more frequent evaluation and additional support from rheumatology providers.

.jpg)
Disclosures: S. Bartlett, Pfizer, Novartis, Merck/MSD, Janssen, AbbVie/Abbott, Organon; o. schieir, None; M. Valois, None; J. Pope, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Eli Lilly, merk, Roche, Seattle Genetics, UCB, Actelion, Amgen, Bayer, Eicos Sciences, Emerald, Gilead, Janssen, Novartis, Pfizer, Sandoz, Sanofi, Boehringer Ingelheim; L. Bessette, AbbVie/Abbott, Amgen, Bristol-Myers Squibb(BMS), Celgene, Eli Lilly, Janssen, Novartis, Pfizer, Sanofi-Genzyme, UCB, Gilead, Merck/MSD, Organon, Roche; G. Boire, AbbVie/Abbott, Bristol-Myers Squibb(BMS), Janssen, Eli Lilly, Merck/MSD, Novartis, Orimed Pharma, Pfizer, Samsung Bioepis, Teva, Viatris, Amgen, Celgene; C. Hitchon, Pfizer Canada, Astra-Zeneca Canada, Pfizer; E. Keystone, AbbVie/Abbott, Amgen, Gilead, Eli Lilly, Merck/MSD, Pfizer, Sanofi, Bristol-Myers Squibb(BMS), Celltrion, Myriad Autoimmune, F. Hoffmann-La Roche Inc, Genentech, Janssen, Sandoz, Samsung Bioepsis, AstraZeneca, Samsung Bioepsis; C. Thorne, AbbVie/Abbott, Amgen, Celgene, CaREBiodam, Centocor, Janssen, Eli Lilly, Novartis, Pfizer, Sanofi, Medexus/Medac, Merck; D. Tin, None; G. Hazlewood, None; V. Bykerk, Amgen, Bristol-Myers Squibb(BMS), Genzyme, Brainstorm, Gilead, Regeneron, UCB, Pfizer, Sanofi, Aventis.

