Back
Poster Session D
Systemic lupus erythematosus (SLE)
Session: (1707–1726) SLE – Animal Models Poster
1721: Protective Effects of the Natural Antioxidant Taxifolin in Models of Lupus and Antiphospholipid Syndrome
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- RA
Ramadan Ali, PhD
University of Michigan
Ann Arobr, MI, United States
Abstract Poster Presenter(s)
Christine Rysenga1, Linda May-Zhang2, Miela Zahavi3, Jason S Knight4 and Ramadan Ali1, 1University of Michigan, Ann Arbor, MI, 2Blue California, Rancho Santa Margarita, CA, 3Universtiy of Michigan, Ann Arbor, MI, 4University of Michigan, Division of Rheumatology, Ann Arbor, MI
Background/Purpose: Taxifolin, also known as dihydroquercetin, is a bioactive flavonoid commonly found in apples, onions, French maritime bark, and milk thistle. Given its potent anti-inflammatory and antioxidant properties, taxifolin has been used as a dietary supplement and studied for ameliorating symptoms in various chronic inflammatory health conditions. Neutrophil extracellular traps (NETs) are important players in inflammatory diseases including lupus and antiphospholipid syndrome (APS). Here, we hypothesized that taxifolin might mitigate NET release (NETosis) in response to lupus- and APS-relevant stimuli. We tested this hypothesis in vitro and in mice.
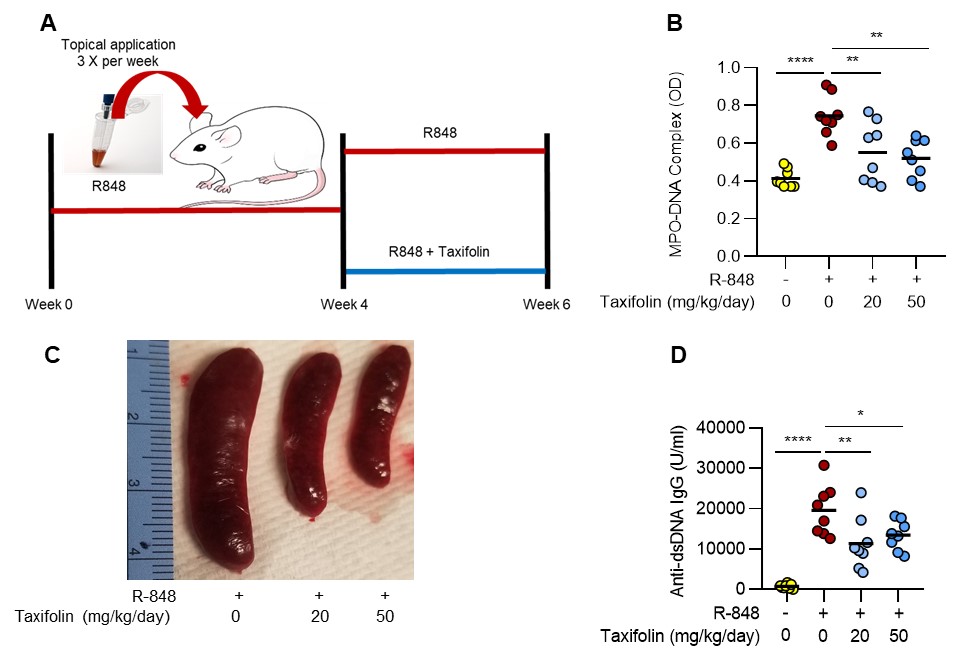
Methods: For in vitro studies, healthy human neutrophils were activated with immune complexes from lupus patients (RNP/anti-RNP) or total IgG fractions from primary APS patients (aPL). NETosis was quantified via the enzymatic activity of NET-associated myeloperoxidase. In vivo, food-grade taxifolin (Taxifolin BC-DHQ®) (20 and 50 mg/kg/day) was given via oral gavage in two models characterized by increased NETosis: TLR7 agonist-induced lupus (BALB/c mice) and the Electrolytic Inferior Vena Cava Model of APS thrombosis (C57BL/6 mice). When the lower dosing strategy extrapolated to humans, it is estimated to be generally recognized as safe (GRAS) for nutritional supplement use.
Results: At concentrations of 1-10 μM, taxifolin reduced NETosis by approximately 50% in neutrophils activated with either RNP/anti-RNP (p=0.0003) or aPL (p=0.007). Taxifolin also potently prevented the neutrophil oxidative burst as hydrogen peroxide production in response to the protein kinase C activator PMA was significantly blunted (p< 0.009). Notably, suppression of NETosis and the oxidative burst by taxifolin could be mitigated by blocking the function of the antioxidant transcription factor Nrf2 (nuclear factor erythroid-2 related factor), which has been shown previously to be activated by taxifolin. In vivo, administration of taxifolin to TLR7 agonist-treated mice (model of lupus) at a dose of 50 mg/kg/day over 2 weeks resulted in a marked reduction of serum NET remnants (reduced by 66%, p=0.001), splenomegaly (50%, p=0.0001), and anti-double-stranded DNA antibodies (33%, p=0.04) (Fig 1); similar reductions were observed with the 20 mg/kg/day dose. In a mouse model of APS thrombosis, mice receiving aPL formed larger thrombi than those receiving control IgG (aPL mean= 6.6mg vs control IgG mean= 3.0mg; p=0.006). When taxifolin was administered to the aPL mice, thrombus weight was significantly reduced (mean= 3.0mg; p=0.001).
Conclusion: We demonstrate for the first time that the natural compound taxifolin decreases lupus- and APS-relevant NETosis through a mechanism that appears to depend on upregulation of the antioxidant transcription factor Nrf2. At the same time, oral administration of taxifolin to mice at doses relevant to those that would be given to humans reduces NETosis in models of lupus and APS thrombosis, while also attenuating other disease-relevant activities such as autoantibody formation and large-vein thrombosis.
Disclosures: C. Rysenga, None; L. May-Zhang, None; M. Zahavi, None; J. Knight, Jazz Pharmaceuticals, Bristol Myers Squibb; R. Ali, None.
Background/Purpose: Taxifolin, also known as dihydroquercetin, is a bioactive flavonoid commonly found in apples, onions, French maritime bark, and milk thistle. Given its potent anti-inflammatory and antioxidant properties, taxifolin has been used as a dietary supplement and studied for ameliorating symptoms in various chronic inflammatory health conditions. Neutrophil extracellular traps (NETs) are important players in inflammatory diseases including lupus and antiphospholipid syndrome (APS). Here, we hypothesized that taxifolin might mitigate NET release (NETosis) in response to lupus- and APS-relevant stimuli. We tested this hypothesis in vitro and in mice.
Methods: For in vitro studies, healthy human neutrophils were activated with immune complexes from lupus patients (RNP/anti-RNP) or total IgG fractions from primary APS patients (aPL). NETosis was quantified via the enzymatic activity of NET-associated myeloperoxidase. In vivo, food-grade taxifolin (Taxifolin BC-DHQ®) (20 and 50 mg/kg/day) was given via oral gavage in two models characterized by increased NETosis: TLR7 agonist-induced lupus (BALB/c mice) and the Electrolytic Inferior Vena Cava Model of APS thrombosis (C57BL/6 mice). When the lower dosing strategy extrapolated to humans, it is estimated to be generally recognized as safe (GRAS) for nutritional supplement use.
Results: At concentrations of 1-10 μM, taxifolin reduced NETosis by approximately 50% in neutrophils activated with either RNP/anti-RNP (p=0.0003) or aPL (p=0.007). Taxifolin also potently prevented the neutrophil oxidative burst as hydrogen peroxide production in response to the protein kinase C activator PMA was significantly blunted (p< 0.009). Notably, suppression of NETosis and the oxidative burst by taxifolin could be mitigated by blocking the function of the antioxidant transcription factor Nrf2 (nuclear factor erythroid-2 related factor), which has been shown previously to be activated by taxifolin. In vivo, administration of taxifolin to TLR7 agonist-treated mice (model of lupus) at a dose of 50 mg/kg/day over 2 weeks resulted in a marked reduction of serum NET remnants (reduced by 66%, p=0.001), splenomegaly (50%, p=0.0001), and anti-double-stranded DNA antibodies (33%, p=0.04) (Fig 1); similar reductions were observed with the 20 mg/kg/day dose. In a mouse model of APS thrombosis, mice receiving aPL formed larger thrombi than those receiving control IgG (aPL mean= 6.6mg vs control IgG mean= 3.0mg; p=0.006). When taxifolin was administered to the aPL mice, thrombus weight was significantly reduced (mean= 3.0mg; p=0.001).
Conclusion: We demonstrate for the first time that the natural compound taxifolin decreases lupus- and APS-relevant NETosis through a mechanism that appears to depend on upregulation of the antioxidant transcription factor Nrf2. At the same time, oral administration of taxifolin to mice at doses relevant to those that would be given to humans reduces NETosis in models of lupus and APS thrombosis, while also attenuating other disease-relevant activities such as autoantibody formation and large-vein thrombosis.

Disclosures: C. Rysenga, None; L. May-Zhang, None; M. Zahavi, None; J. Knight, Jazz Pharmaceuticals, Bristol Myers Squibb; R. Ali, None.

