Back
Poster Session B
Reproductive health
Session: (0939–0969) Reproductive Issues in Rheumatic Disorders Poster
0959: Assessing the Association Between Hydroxychloroquine and Preeclampsia Risk in SLE Pregnancies Using Administrative Claims Data
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- AR
Amadeia Rector, MPH
Stanford University
San Francisco, CA, United States
Abstract Poster Presenter(s)
Amadeia Rector1, Ivana Marić2, Yashaar Chaichian3, Eliza Chakravarty4, Miranda Cantu5, Michael Weisman6, Gary Shaw7, Maurice Druzin7 and Julia Simard1, 1Stanford University School of Medicine, Palo Alto, CA, 2Stanford University, Stanford, 3Stanford University, Stanford, CA, 4Oklahoma Medical Research Foundation, Oklahoma City, OK, 5Global Lupus Support Group, Portage, MI, 6Adjunct Professor of Medicine, Stanford University; Distinguished Professor of Medicine Emeritus, David Geffen School of Medicine at UCLA, Los Angeles, CA, 7Stanford University School of Medicine, Palo Alto
Background/Purpose: Preeclampsia risk is higher among pregnancies in patients with systemic lupus erythematosus (SLE). Hydroxychloroquine (HCQ), a common first-line treatment, is encouraged in SLE pregnancies as lupus-associated flares may increase risk of adverse pregnancy outcomes. HCQ may interrupt preeclampsia pathogenesis by mitigating oxidative stress and targeting toll-like receptors to prevent pro-inflammatory cytokine production. We investigated HCQ use and preeclampsia risk in patients with SLE.
Methods: We studied 1096 singleton liveborn pregnancies among 1043 patients with SLE continuously enrolled in private health insurance (IBM® MarketScan® Commercial Database) from 3 months before last menstrual period to pregnancy end (2007-2016). Modified Poisson models estimated risk ratios (RRs) and 95% confidence intervals (95% CIs) by parity. Multiple approaches used propensity scores (PS) to account for confounders (e.g., age, obesity, pregestational hypertension, prior renal manifestations, antiphospholipid antibodies/antiphospholipid syndrome (aPL/APS), pre-pregnancy steroids, and azathioprine use). Stratified analyses examined effect modification by multiple factors including flare during pregnancy, aPL/APS, prior renal manifestations, pregestational hypertension, and prior preeclampsia. Multiple sensitivity analyses redefined exposure to account for possible nonadherence.
Results: 15% of pregnancies were diagnosed with preeclampsia and 9% delivered preterm. 52% of pregnancies filled HCQ at least once. The HCQ-exposed pregnancies had more flare during pregnancy, pre-pregnancy steroid use, prior renal manifestations, and azathioprine use. We did not observe an association between HCQ and preeclampsia among nulliparous (RR 1.32, 95% CI 0.87, 2.00) nor parous pregnancies (RR 1.25, 95% CI 0.84, 1.85). Additional control for confounding via multiple methods decreased the RRs towards the null in primary and sensitivity analyses (nulliparous: PS-adj RR 1.12, 95% CI 0.71, 1.79 and parous: PS-adj RR 1.09, 95% CI 0.72, 1.64). Among pregnancies with prior preeclampsia, the RR was further attenuated (PS-adj RR 0.85, 95% CI 0.26, 2.80).
Conclusion: Growing evidence suggests that HCQ may protect against preeclampsia in SLE pregnancy as well as in patients with APS. Using a large insurance-based database we found a null association but noted evidence of higher disease activity among HCQ-exposed pregnancies. Data on parity and prior preeclampsia in claims data are often incomplete and prone to misclassification. Further studies leveraging large population-based data, prospective collection, and trials may better characterize how HCQ may influence preeclampsia risk in SLE. Acknowledgments: Data access for this project was provided by the Stanford Center for Population Health Sciences (PHS) Data Core. Disclaimer: IBM Watson Health and MarketScan are trademarks of IBM Corporation in the United States, other countries or both.
.jpeg) Figure 1. Flowchart of SLE pregnancy cohort, 2007-2016. SLE = systemic lupus erythematosus, LB = livebirth, SB = stillbirth, MB = mixed birth
Figure 1. Flowchart of SLE pregnancy cohort, 2007-2016. SLE = systemic lupus erythematosus, LB = livebirth, SB = stillbirth, MB = mixed birth
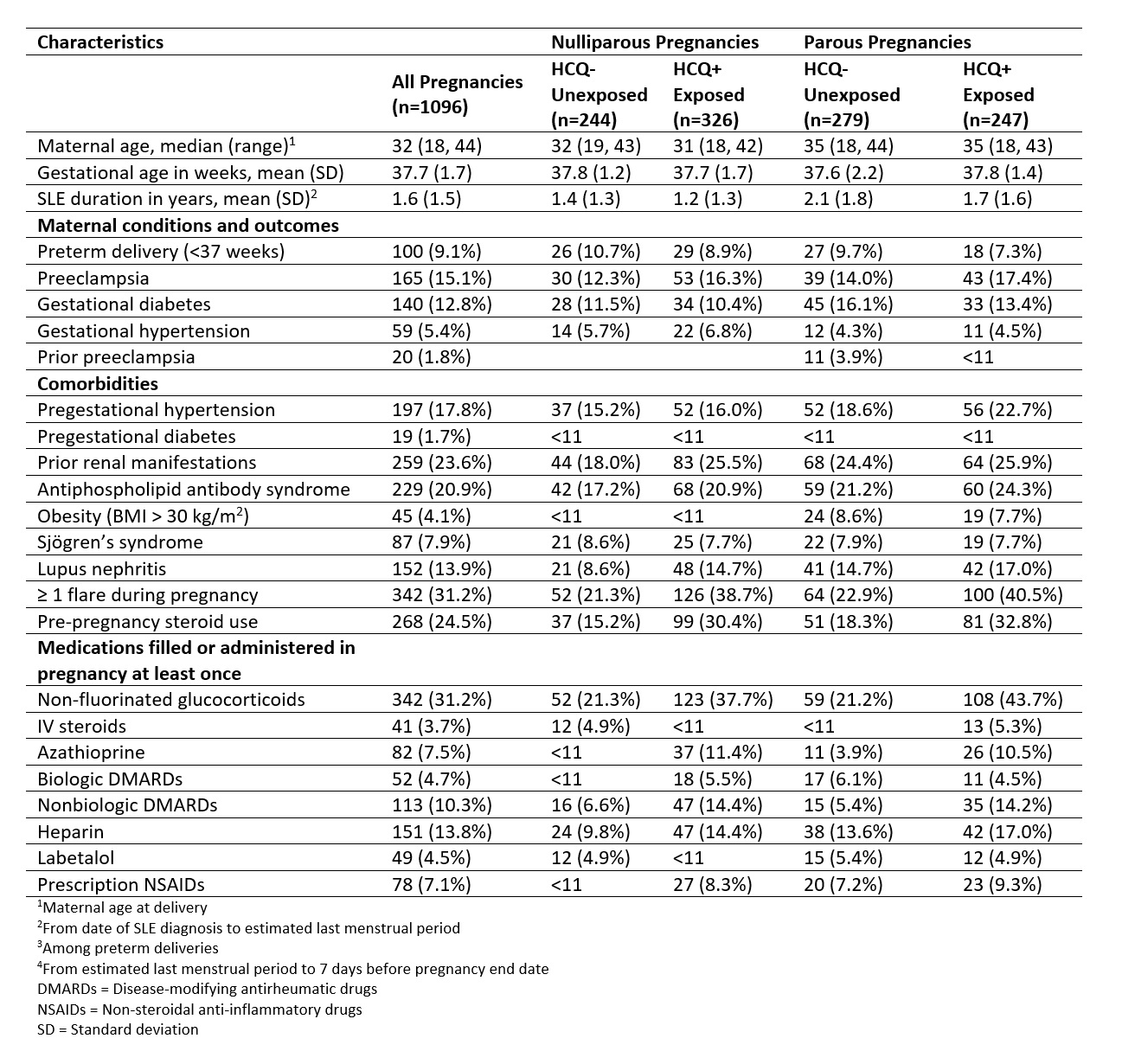 Table 1. Demographics and clinical characteristics for liveborn pregnancies in patients with SLE by HCQ exposure (IBM MarketScan Research Databases, 2007-2016). HCQ exposure is defined as one or more fills from 3 months before LMP to the end of the first trimester.
Table 1. Demographics and clinical characteristics for liveborn pregnancies in patients with SLE by HCQ exposure (IBM MarketScan Research Databases, 2007-2016). HCQ exposure is defined as one or more fills from 3 months before LMP to the end of the first trimester.
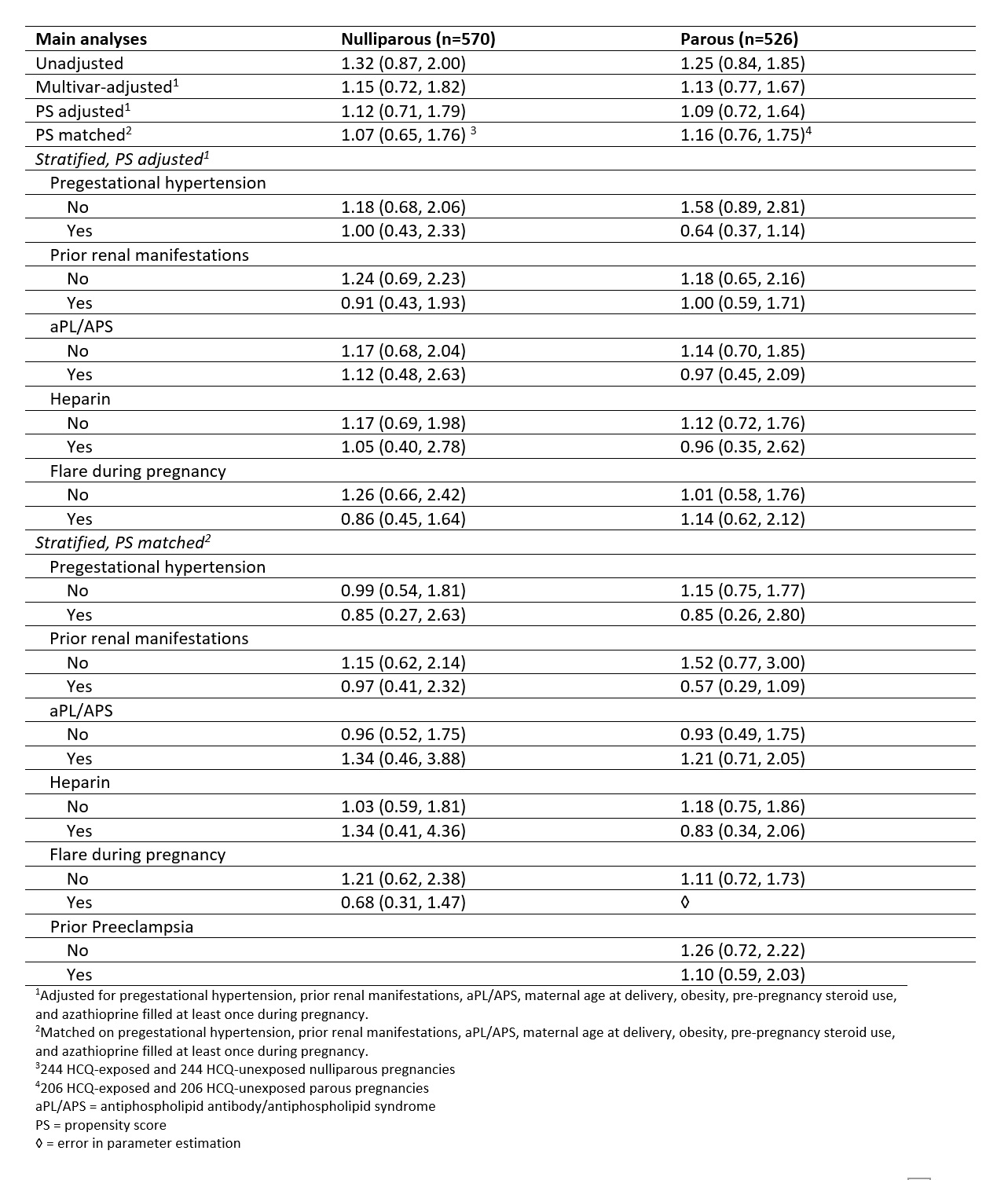 Table 2. Relative risks of preeclampsia among nulliparous and parous singleton SLE pregnancies, presented as RR (95% CI).
Table 2. Relative risks of preeclampsia among nulliparous and parous singleton SLE pregnancies, presented as RR (95% CI).
Disclosures: A. Rector, None; I. Marić, None; Y. Chaichian, Amgen, Eli Lilly, Boehringer-Ingelheim; E. Chakravarty, None; M. Cantu, None; M. Weisman, None; G. Shaw, None; M. Druzin, None; J. Simard, None.
Background/Purpose: Preeclampsia risk is higher among pregnancies in patients with systemic lupus erythematosus (SLE). Hydroxychloroquine (HCQ), a common first-line treatment, is encouraged in SLE pregnancies as lupus-associated flares may increase risk of adverse pregnancy outcomes. HCQ may interrupt preeclampsia pathogenesis by mitigating oxidative stress and targeting toll-like receptors to prevent pro-inflammatory cytokine production. We investigated HCQ use and preeclampsia risk in patients with SLE.
Methods: We studied 1096 singleton liveborn pregnancies among 1043 patients with SLE continuously enrolled in private health insurance (IBM® MarketScan® Commercial Database) from 3 months before last menstrual period to pregnancy end (2007-2016). Modified Poisson models estimated risk ratios (RRs) and 95% confidence intervals (95% CIs) by parity. Multiple approaches used propensity scores (PS) to account for confounders (e.g., age, obesity, pregestational hypertension, prior renal manifestations, antiphospholipid antibodies/antiphospholipid syndrome (aPL/APS), pre-pregnancy steroids, and azathioprine use). Stratified analyses examined effect modification by multiple factors including flare during pregnancy, aPL/APS, prior renal manifestations, pregestational hypertension, and prior preeclampsia. Multiple sensitivity analyses redefined exposure to account for possible nonadherence.
Results: 15% of pregnancies were diagnosed with preeclampsia and 9% delivered preterm. 52% of pregnancies filled HCQ at least once. The HCQ-exposed pregnancies had more flare during pregnancy, pre-pregnancy steroid use, prior renal manifestations, and azathioprine use. We did not observe an association between HCQ and preeclampsia among nulliparous (RR 1.32, 95% CI 0.87, 2.00) nor parous pregnancies (RR 1.25, 95% CI 0.84, 1.85). Additional control for confounding via multiple methods decreased the RRs towards the null in primary and sensitivity analyses (nulliparous: PS-adj RR 1.12, 95% CI 0.71, 1.79 and parous: PS-adj RR 1.09, 95% CI 0.72, 1.64). Among pregnancies with prior preeclampsia, the RR was further attenuated (PS-adj RR 0.85, 95% CI 0.26, 2.80).
Conclusion: Growing evidence suggests that HCQ may protect against preeclampsia in SLE pregnancy as well as in patients with APS. Using a large insurance-based database we found a null association but noted evidence of higher disease activity among HCQ-exposed pregnancies. Data on parity and prior preeclampsia in claims data are often incomplete and prone to misclassification. Further studies leveraging large population-based data, prospective collection, and trials may better characterize how HCQ may influence preeclampsia risk in SLE. Acknowledgments: Data access for this project was provided by the Stanford Center for Population Health Sciences (PHS) Data Core. Disclaimer: IBM Watson Health and MarketScan are trademarks of IBM Corporation in the United States, other countries or both.
.jpeg) Figure 1. Flowchart of SLE pregnancy cohort, 2007-2016. SLE = systemic lupus erythematosus, LB = livebirth, SB = stillbirth, MB = mixed birth
Figure 1. Flowchart of SLE pregnancy cohort, 2007-2016. SLE = systemic lupus erythematosus, LB = livebirth, SB = stillbirth, MB = mixed birth Table 1. Demographics and clinical characteristics for liveborn pregnancies in patients with SLE by HCQ exposure (IBM MarketScan Research Databases, 2007-2016). HCQ exposure is defined as one or more fills from 3 months before LMP to the end of the first trimester.
Table 1. Demographics and clinical characteristics for liveborn pregnancies in patients with SLE by HCQ exposure (IBM MarketScan Research Databases, 2007-2016). HCQ exposure is defined as one or more fills from 3 months before LMP to the end of the first trimester. Table 2. Relative risks of preeclampsia among nulliparous and parous singleton SLE pregnancies, presented as RR (95% CI).
Table 2. Relative risks of preeclampsia among nulliparous and parous singleton SLE pregnancies, presented as RR (95% CI).Disclosures: A. Rector, None; I. Marić, None; Y. Chaichian, Amgen, Eli Lilly, Boehringer-Ingelheim; E. Chakravarty, None; M. Cantu, None; M. Weisman, None; G. Shaw, None; M. Druzin, None; J. Simard, None.

