Back
Poster Session B
Spondyloarthritis (SpA) including psoriatic arthritis (PsA)
Session: (1004–1034) Spondyloarthritis Including PsA – Diagnosis, Manifestations, and Outcomes Poster II
1019: Association Between Stringent Clinical Measures of Disease Activity and Clinically Meaningful Improvements in Patient-Reported Outcomes in Psoriatic Arthritis: Results from KEEPsAKE 1 and 2 Clinical Trials
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
.png)
Alexis Ogdie, MD
Rheumatologist, Associate Professor of Medicine
University of Pennsylvania
Philadelphia, PA, United States
Abstract Poster Presenter(s)
Alexis Ogdie1, Joseph Merola2, Roberto Ranza3, Ahmed Soliman4, Manish Mittal4, Byron Padilla5, Sandra Ciecinski6, Priscila Nakasato7 and Laure Gossec8, 1Department of Medicine, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA, 2Brigham and Women's Hospital, Harvard Medical School, Boston, MA, 3Serviço de Reumatología, Universidade Federal de Uberlândia, Uberlândia, Brazil, 4AbbVie, Inc., North Chicago, IL, 5AbbVie, Inc., Waukegan, IL, 6AbbVie, Inc., Mettawa, IL, 7AbbVie Inc, Toronto, ON, Canada, 8Sorbonne Université, Paris, France
Background/Purpose: Assessment of disease activity in PsA patients is important to evaluate therapeutic response in clinical trials. Patient-reported outcomes (PROs) are evaluated to assess benefits of therapy from the patient perspective. The association between stringent measures of clinical disease control and clinically meaningful improvements in PROs remains to be established. We examined the relationship between achieving stringent disease control and improvement in PROs in PsA patients.
Methods: Post-hoc analysis of data from two phase 3 trials, KEEPsAKE 1 (NCT03675308) and 2 (NCT03671148), comparing risankizumab to placebo in patients with active PsA and an inadequate response to csDMARDs and/or biologics. Measures of stringent disease control included achievement of minimal disease activity (MDA, achievement of ≥5 of the following: tender or swollen joint count ≤1, Psoriasis Area Severity Index ≤1 or body surface area ≤3%, patient's assessment of pain ≤15 out of 100, patient's global assessment of disease activity ≤20 out of 100, HAQ-Disability Index (HAQ-DI) ≤0.5, or tender entheseal points ≤1), low disease activity (LDA) based on Disease Activity Index in PsA (DAPSA score ≤14), and ACR50 response. PROs included patient's VAS assessment of pain, HAQ-DI, Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, 36-item Short-Form Health Survey (SF-36) Physical (PCS) and Mental (MCS) Component Summary scores, and overall work productivity loss (Work Productivity and Activity Impairment questionnaire; WPAI-PsA). The percentage of patients achieving minimal clinically important differences (MCID) in PROs was determined in patients who achieved (responders) vs those who did not achieve (non-responders) stringent disease control at Week 24. Comparisons between groups were assessed using logistic regression controlling for baseline score of PRO analyzed and stratification factors of current use of csDMARDs (0 vs ≥1), number of prior biologic therapies (0 vs ≥1), and extent of psoriasis (≥3% BSA or < 3% BSA) at baseline.
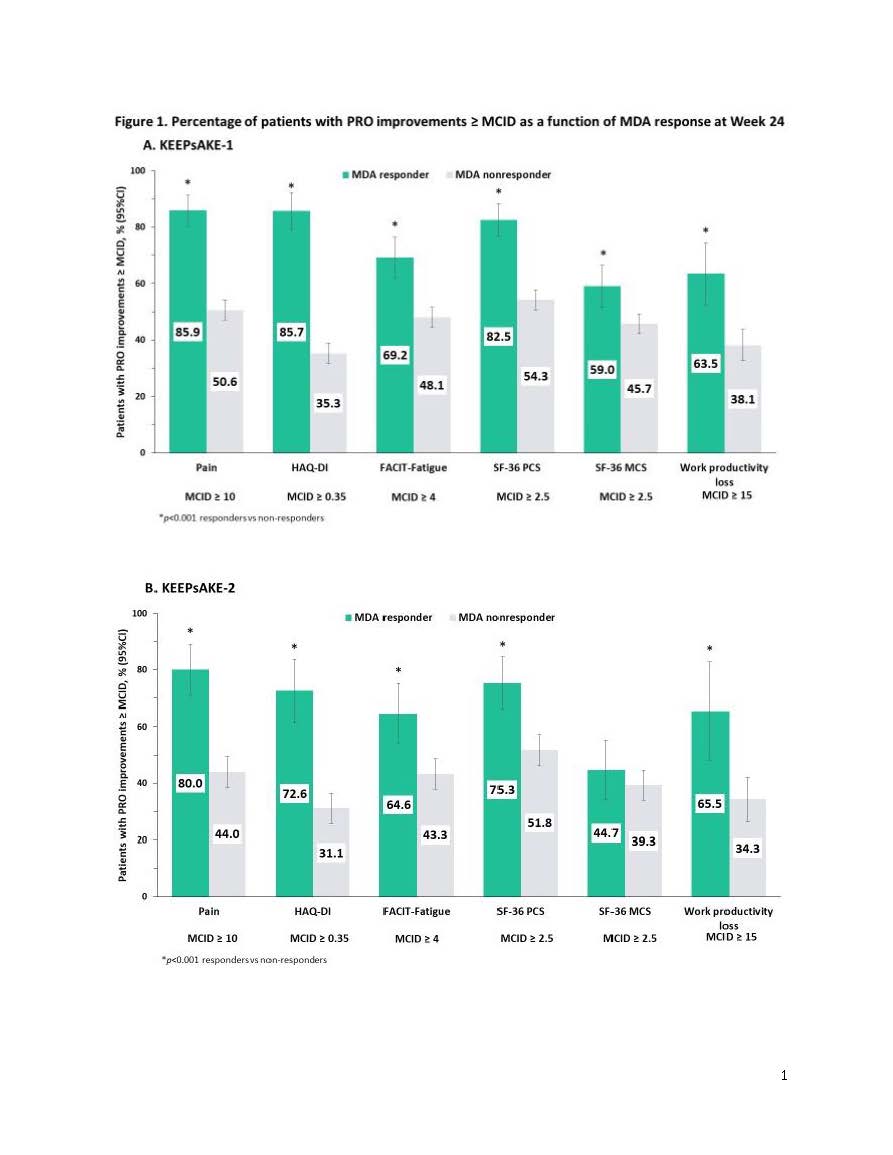
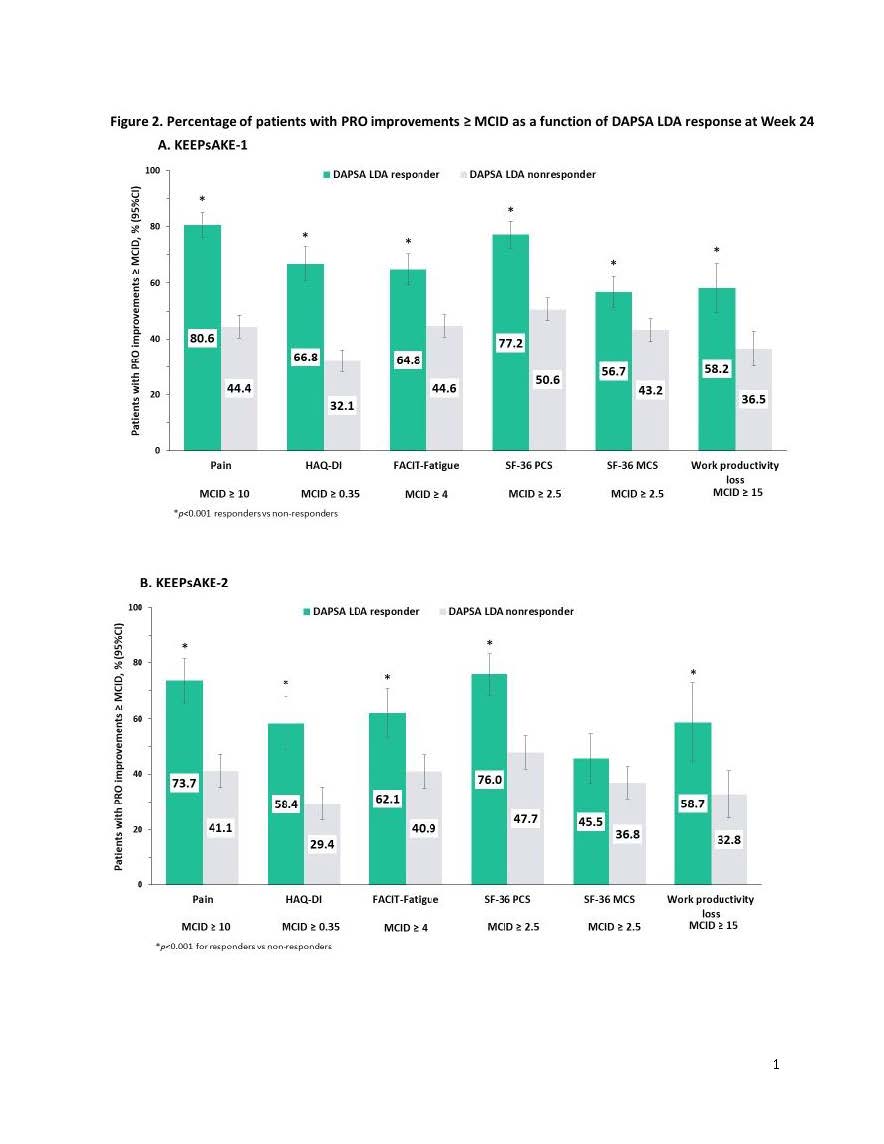
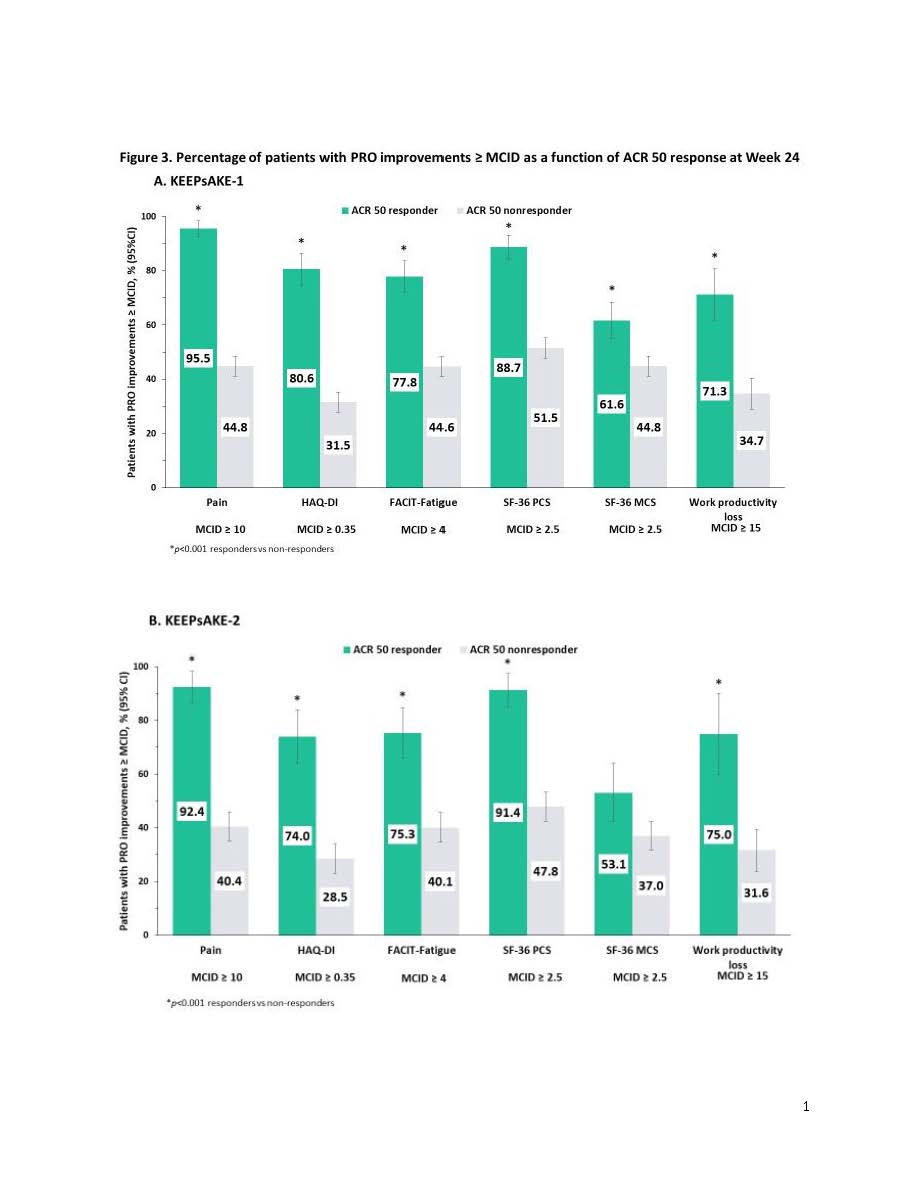
Results: At Week 24, 18%, 34% and 22% of patients in KEEPsAKE 1 and 21%, 31% and 20% in KEEPsAKE 2 were MDA, DAPSA LDA and ACR50 responders, respectively. Percentages of patients with improvements ≥MCID in pain, HAQ-DI, FACIT-Fatigue, SF-36 PCS and MCS, as well as work productivity loss scores were significantly greater (p< 0.001) in MDA responders vs non-responders in KEEPsAKE-1 (range: 63.5% to 85.9% vs 35.3% to 54.3%; Figure 1A) and KEEPsAKE-2, except SF-36 MCS (range: 65.5% to 80.0% vs 31.1% to 51.8%; Figure 1B). Results (p< 0.001) were also significant for DAPSA LDA (KEEPsAKE-1: 58.2% to 80.6% vs 32.1% to 50.6%; KEEPsAKE-2: 58.4% to 76.0% vs 29.4% to 47.7%; Figure 2A & B, respectively) and ACR50 (KEEPsAKE-1: 71.3% to 95.5% vs 31.5% to 51.5%; KEEPsAKE-2: 74.0% to 92.4% vs 28.5% to 47.8%; Figure 3A & B, respectively) responders vs non-responders.
Conclusion: Significantly more PsA patients who were MDA, DAPSA LDA, or ACR50 responders vs non-responders reported clinically meaningful improvements across a broad range of PROs including pain, physical function, fatigue, and work productivity demonstrating the value of achieving stringent clinical measures of disease control in PsA.
Disclosures: A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB; J. Merola, Novartis, Pfizer, Sun Pharma, UCB Pharma, Bristol-Myers Squibb(BMS), AbbVie, Dermavant, Eli Lilly, Janssen, Arena, Biogen; R. Ranza, AbbVie, Janssen, Novartis, Pfizer; A. Soliman, AbbVie; M. Mittal, AbbVie; B. Padilla, AbbVie; S. Ciecinski, AbbVie; P. Nakasato, AbbVie; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD.
Background/Purpose: Assessment of disease activity in PsA patients is important to evaluate therapeutic response in clinical trials. Patient-reported outcomes (PROs) are evaluated to assess benefits of therapy from the patient perspective. The association between stringent measures of clinical disease control and clinically meaningful improvements in PROs remains to be established. We examined the relationship between achieving stringent disease control and improvement in PROs in PsA patients.
Methods: Post-hoc analysis of data from two phase 3 trials, KEEPsAKE 1 (NCT03675308) and 2 (NCT03671148), comparing risankizumab to placebo in patients with active PsA and an inadequate response to csDMARDs and/or biologics. Measures of stringent disease control included achievement of minimal disease activity (MDA, achievement of ≥5 of the following: tender or swollen joint count ≤1, Psoriasis Area Severity Index ≤1 or body surface area ≤3%, patient's assessment of pain ≤15 out of 100, patient's global assessment of disease activity ≤20 out of 100, HAQ-Disability Index (HAQ-DI) ≤0.5, or tender entheseal points ≤1), low disease activity (LDA) based on Disease Activity Index in PsA (DAPSA score ≤14), and ACR50 response. PROs included patient's VAS assessment of pain, HAQ-DI, Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, 36-item Short-Form Health Survey (SF-36) Physical (PCS) and Mental (MCS) Component Summary scores, and overall work productivity loss (Work Productivity and Activity Impairment questionnaire; WPAI-PsA). The percentage of patients achieving minimal clinically important differences (MCID) in PROs was determined in patients who achieved (responders) vs those who did not achieve (non-responders) stringent disease control at Week 24. Comparisons between groups were assessed using logistic regression controlling for baseline score of PRO analyzed and stratification factors of current use of csDMARDs (0 vs ≥1), number of prior biologic therapies (0 vs ≥1), and extent of psoriasis (≥3% BSA or < 3% BSA) at baseline.
Results: At Week 24, 18%, 34% and 22% of patients in KEEPsAKE 1 and 21%, 31% and 20% in KEEPsAKE 2 were MDA, DAPSA LDA and ACR50 responders, respectively. Percentages of patients with improvements ≥MCID in pain, HAQ-DI, FACIT-Fatigue, SF-36 PCS and MCS, as well as work productivity loss scores were significantly greater (p< 0.001) in MDA responders vs non-responders in KEEPsAKE-1 (range: 63.5% to 85.9% vs 35.3% to 54.3%; Figure 1A) and KEEPsAKE-2, except SF-36 MCS (range: 65.5% to 80.0% vs 31.1% to 51.8%; Figure 1B). Results (p< 0.001) were also significant for DAPSA LDA (KEEPsAKE-1: 58.2% to 80.6% vs 32.1% to 50.6%; KEEPsAKE-2: 58.4% to 76.0% vs 29.4% to 47.7%; Figure 2A & B, respectively) and ACR50 (KEEPsAKE-1: 71.3% to 95.5% vs 31.5% to 51.5%; KEEPsAKE-2: 74.0% to 92.4% vs 28.5% to 47.8%; Figure 3A & B, respectively) responders vs non-responders.
Conclusion: Significantly more PsA patients who were MDA, DAPSA LDA, or ACR50 responders vs non-responders reported clinically meaningful improvements across a broad range of PROs including pain, physical function, fatigue, and work productivity demonstrating the value of achieving stringent clinical measures of disease control in PsA.



Disclosures: A. Ogdie, AbbVie, Amgen, Novartis, Pfizer Inc, Bristol-Myers Squibb, Celgene, Janssen, CorEvitas, Gilead Sciences, Eli Lilly, GlaxoSmithKline, Happify Health, UCB; J. Merola, Novartis, Pfizer, Sun Pharma, UCB Pharma, Bristol-Myers Squibb(BMS), AbbVie, Dermavant, Eli Lilly, Janssen, Arena, Biogen; R. Ranza, AbbVie, Janssen, Novartis, Pfizer; A. Soliman, AbbVie; M. Mittal, AbbVie; B. Padilla, AbbVie; S. Ciecinski, AbbVie; P. Nakasato, AbbVie; L. Gossec, Amgen, Lilly, Pfizer, Sandoz, UCB Pharma, AbbVie, Bristol Myers Squibb, Gilead, Janssen, Novartis, Samsung Bioepis, Sanofi-Aventis, Galapagos, GlaxoSmithKlein (GSK), Celltrion, MSD.

