Back
Poster Session D
Immunobiology
Session: (1681–1706) Innate Immunity Poster: Basic and Translational Science
1693: Skin Pigmentation Association with Regulation of Cyclic-GMP-AMP Following Skin Exposure to UV Light
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JA
Jie An, PhD
University of Washington
Seattle, WA, United States
Abstract Poster Presenter(s)
Jie An1, Sladjana Skopelja-Gardner2, Xizhang Sun1, Lena Tanaka1 and Keith Elkon1, 1University of Washington, Seattle, WA, 2Dartmouth Hitchcock Medical Center, Lebanon, NH
Background/Purpose: Amongst the most important cytosolic DNA sensors is Cyclic GMP-AMP synthase (cGAS). Binding of DNA to cGAS results in the synthesis of the cyclic dinucleotide second messenger, cGAMP. cGAMP binding to the adapter protein, STING, culminates in the synthesis of IFN-β. cGAMP is regulated by export from cells into the extracellular space where it can be hydrolyzed by the enzyme, ENPP1 or imported into neighboring cells. To determine whether cGAMP acts as an immunotransmitter to allow spreading of the IFN-I response following skin exposure to UV light, we inhibited ENPP1 locally in the skin and also tested the IFN-I response of mice deficient in ENPP1 (ENPP1 KO). Considering that ENPP1 is not expressed by keratinocytes but is expressed by melanocytes and that the prevalence of photosensitivity is greater in white (~70%) compared to black (~20%) SLE patients (PMID: 2787184), we also explored the relationship between ENPP1 expression and the IFN-I response to UVB in pigmented and non-pigmented regions of C57BL/6 (B6) mouse skin.
Methods: B6 mice were exposed to a single dose of UVB (500 mJ/cm2) and biopsies taken before and at 24 hours after exposure. Expression of IFN stimulated genes (ISGs) and other inflammatory cytokines were measured by qPCR. To examine the effects of reducing the extracellular hydrolysis of cGAMP, we used a chemical inhibitor of ENPP1, STF1084 30 minutes prior to UV exposure. In addition, we asked whether IFN responses were altered in mice that were deficient in ENPP1 (KO mice) following exposure to UVB. We compared ENPP1 expression in pigmented and non-pigmented regions obtained from B6 skin in CD45- skin cells by flow cytometry using an anti-mouse ENPP1 antibody. Statistical significance between groups was determined by Student's t-test.
Results: In experiments to address the role of extracellular cGAMP, we observed that local application of the ENPP1 inhibitor, STF 1084, significantly increased ISGs in non-UV exposed skin (Fig. 1A). When ENPP1 KO (n=7) and WT mice (n=4) mice were exposed to UVB, the mean IFN score in the skin was 4.1 fold higher in the ENPP1 KO compared to controls (Fig. 1B). We observed that a significantly higher percentage of skin cells from pigmented regions expressed ENPP1 compared to non-pigmented regions from the same B6 mice (~30% vs ~6 % of CD45- cells, respectively, Fig 2A). When pigmented versus non-pigmented regions of skin were compared for ISG responses we found ISGs to be consistently higher in non-pigmented skin (Fig. 2B).
Conclusion: Inhibition or deficiency of ENPP1 leads to greater IFN-I responses to UVB indicating that this enzyme is required to degrade cGAMP in the extracellular space and prevent spreading of the IFN-I response. We confirmed that ENPP1 was highly expressed on non-immune (CD45-) skin cells and that expression was higher in pigmented compared to non-pigmented regions. Preliminary results suggest that UV exposure of the pigmented regions were associated with lower ISGs compared to non-pigmented regions of skin. Therefore, in addition to the roles of melanin in light absorption and scattering, the higher ENPP1 expression may contribute to protection from photosensitivity observed in black SLE patients.
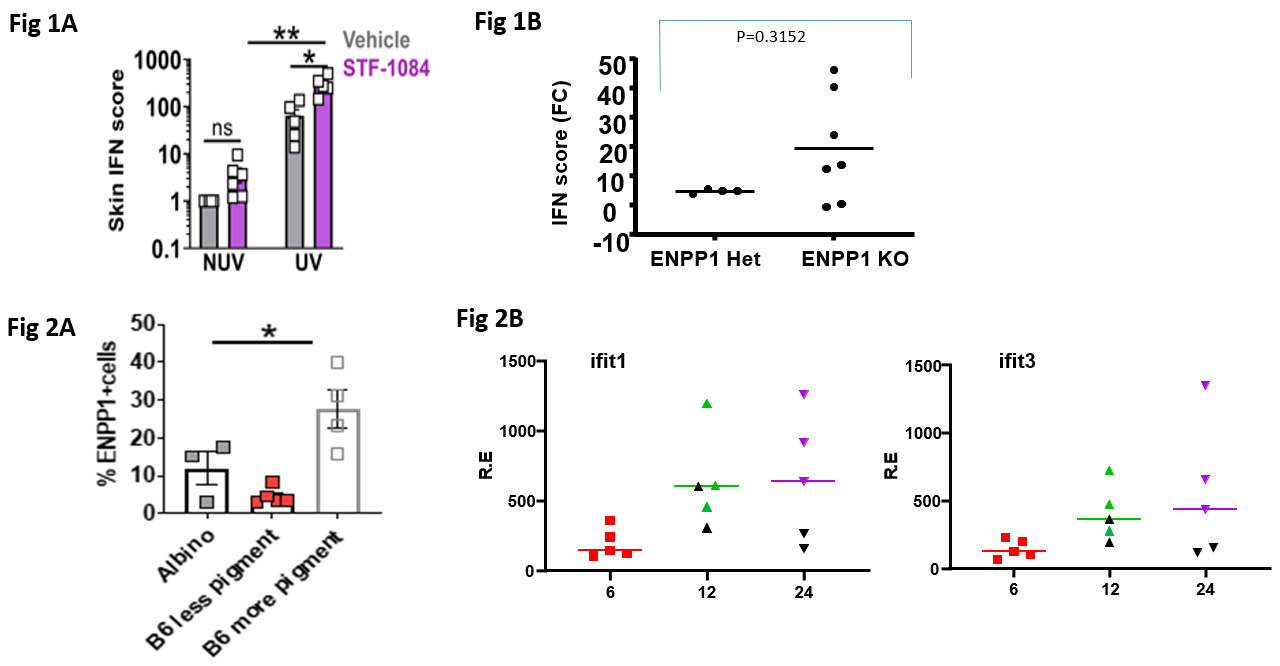 Figure 1: (A) Effect of local pre-application of the ENPP1 inhibitor, STF 1084, to mouse skin prior to UVB. (B) Effect of ENPP1 deficiency on UVB induced IFN responses. Figure 2. (A) Expression of ENPP1 on skin cells from pigmented and non-pigmented regions of skin. (B) Expression of ISGs (Ifit1 and Ifit3) in non-pigmented (green or purple symbols) compared to pigmented regions (black symbols) of skin following UVB exposure.
Figure 1: (A) Effect of local pre-application of the ENPP1 inhibitor, STF 1084, to mouse skin prior to UVB. (B) Effect of ENPP1 deficiency on UVB induced IFN responses. Figure 2. (A) Expression of ENPP1 on skin cells from pigmented and non-pigmented regions of skin. (B) Expression of ISGs (Ifit1 and Ifit3) in non-pigmented (green or purple symbols) compared to pigmented regions (black symbols) of skin following UVB exposure.
Disclosures: J. An, None; S. Skopelja-Gardner, None; X. Sun, None; L. Tanaka, None; K. Elkon, None.
Background/Purpose: Amongst the most important cytosolic DNA sensors is Cyclic GMP-AMP synthase (cGAS). Binding of DNA to cGAS results in the synthesis of the cyclic dinucleotide second messenger, cGAMP. cGAMP binding to the adapter protein, STING, culminates in the synthesis of IFN-β. cGAMP is regulated by export from cells into the extracellular space where it can be hydrolyzed by the enzyme, ENPP1 or imported into neighboring cells. To determine whether cGAMP acts as an immunotransmitter to allow spreading of the IFN-I response following skin exposure to UV light, we inhibited ENPP1 locally in the skin and also tested the IFN-I response of mice deficient in ENPP1 (ENPP1 KO). Considering that ENPP1 is not expressed by keratinocytes but is expressed by melanocytes and that the prevalence of photosensitivity is greater in white (~70%) compared to black (~20%) SLE patients (PMID: 2787184), we also explored the relationship between ENPP1 expression and the IFN-I response to UVB in pigmented and non-pigmented regions of C57BL/6 (B6) mouse skin.
Methods: B6 mice were exposed to a single dose of UVB (500 mJ/cm2) and biopsies taken before and at 24 hours after exposure. Expression of IFN stimulated genes (ISGs) and other inflammatory cytokines were measured by qPCR. To examine the effects of reducing the extracellular hydrolysis of cGAMP, we used a chemical inhibitor of ENPP1, STF1084 30 minutes prior to UV exposure. In addition, we asked whether IFN responses were altered in mice that were deficient in ENPP1 (KO mice) following exposure to UVB. We compared ENPP1 expression in pigmented and non-pigmented regions obtained from B6 skin in CD45- skin cells by flow cytometry using an anti-mouse ENPP1 antibody. Statistical significance between groups was determined by Student's t-test.
Results: In experiments to address the role of extracellular cGAMP, we observed that local application of the ENPP1 inhibitor, STF 1084, significantly increased ISGs in non-UV exposed skin (Fig. 1A). When ENPP1 KO (n=7) and WT mice (n=4) mice were exposed to UVB, the mean IFN score in the skin was 4.1 fold higher in the ENPP1 KO compared to controls (Fig. 1B). We observed that a significantly higher percentage of skin cells from pigmented regions expressed ENPP1 compared to non-pigmented regions from the same B6 mice (~30% vs ~6 % of CD45- cells, respectively, Fig 2A). When pigmented versus non-pigmented regions of skin were compared for ISG responses we found ISGs to be consistently higher in non-pigmented skin (Fig. 2B).
Conclusion: Inhibition or deficiency of ENPP1 leads to greater IFN-I responses to UVB indicating that this enzyme is required to degrade cGAMP in the extracellular space and prevent spreading of the IFN-I response. We confirmed that ENPP1 was highly expressed on non-immune (CD45-) skin cells and that expression was higher in pigmented compared to non-pigmented regions. Preliminary results suggest that UV exposure of the pigmented regions were associated with lower ISGs compared to non-pigmented regions of skin. Therefore, in addition to the roles of melanin in light absorption and scattering, the higher ENPP1 expression may contribute to protection from photosensitivity observed in black SLE patients.
 Figure 1: (A) Effect of local pre-application of the ENPP1 inhibitor, STF 1084, to mouse skin prior to UVB. (B) Effect of ENPP1 deficiency on UVB induced IFN responses. Figure 2. (A) Expression of ENPP1 on skin cells from pigmented and non-pigmented regions of skin. (B) Expression of ISGs (Ifit1 and Ifit3) in non-pigmented (green or purple symbols) compared to pigmented regions (black symbols) of skin following UVB exposure.
Figure 1: (A) Effect of local pre-application of the ENPP1 inhibitor, STF 1084, to mouse skin prior to UVB. (B) Effect of ENPP1 deficiency on UVB induced IFN responses. Figure 2. (A) Expression of ENPP1 on skin cells from pigmented and non-pigmented regions of skin. (B) Expression of ISGs (Ifit1 and Ifit3) in non-pigmented (green or purple symbols) compared to pigmented regions (black symbols) of skin following UVB exposure.Disclosures: J. An, None; S. Skopelja-Gardner, None; X. Sun, None; L. Tanaka, None; K. Elkon, None.

