Back
Poster Session D
Immunobiology
Session: (1727–1749) T Cell Biology and Targets in Autoimmune and Inflammatory Disease Poster
1729: SLAMF4+ CCR5+ Effector Memory CD4+ T Cells Are Polyfunctional, Resistant to Exhaustion, and Expanded in Rheumatoid Arthritis
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- jB
jerome Biton, PhD
Université Sorbonne Paris Nord, Inserm UMR1125
Bobigny, France
Abstract Poster Presenter(s)
Mégane Lacaud1, Houda-Ghozlane Bouzidi1, Magali Breckler2, Delphine Lemeiter2, Johanna Sigaux3, Luca Semerano4, Marie-Christophe Boissier5, Natacha Bessis6 and Jerome Biton7, 1Sorbonne Paris Nord University, Inserm U1125, Bobigny, France, 2Sorbonne Paris Nord University, Inserm UMR1125, Bobigny, France, 3Avicenne Hospital, Sorbonne Paris Nord University, Inserm UMR1125, Bobigny, France, 4Hopital Avicenne - AP-HP, Sorbonne Paris Nord University, INSERM UMR1125, Bobigny Cedex, France, 5INSERM UMR 1125, Sorbonne Paris Nord University,Rheumatology Dpt, APHP, GHUPSSD, Avicenne Hosp, Bobigny, France, 6INSERM 1125 and University Sorbonne Paris Nord, Bobigny, France, 7INSERM UMR 1125, Sorbonne Paris Nord University, Bobigny, France
Background/Purpose: CD4+ Foxp3- conventional T cells (Tconv) play a key role in the inflammatory process involved in rheumatoid arthritis (RA). Usually, chronic stimulation of T cells promotes their exhaustion. This state of anergy is induced by the co-expression of different inhibitory receptors (PD-1, TIM-3...). However, it is becoming increasingly clear that in various autoimmune diseases, chronic Tconv stimulation does not induce a sufficient level of exhaustion to inhibit their pathological response. Overexpression of activating receptors by T cells, counterbalancing that of their inhibitory counterparts, may allow them to maintain their functions in the presence of chronic activation. Among the receptors potentially capable of upregulating the Tconv response, SLAMFs (Signaling lymphocytic activation molecule) represent a family of nine receptors. In RA, our objectives were to determine whether SLAMF receptors are involved in the establishment of the Tconv pro-inflammatory response and whether these receptors represent a protection against Tconv exhaustion.
Methods: We immunophenotyped blood T cells from RA patients (n=64) and from healthy donors (HD) (n=14) using a 13-marker flow cytometry panel. The expression of SLAMF receptors was determine among four Tconv subpopulations with different activation status (naive, central memory, effector memory and terminally differentiated effector CD4+ T cells). To assess the inflammatory tropism and functionality of different Tconv subpopulations, CCR5 expression as well as TNF-α and IFN-γ secretion were studied, respectively. Comparisons between RA patient samples and HD were performed using a Kruskal–Wallis test followed by an appropriate correction. Association between Tconv subpopulation frequency and RA activity was assessed by Spearman correlations and R software was used for multiparametric graphical representations.
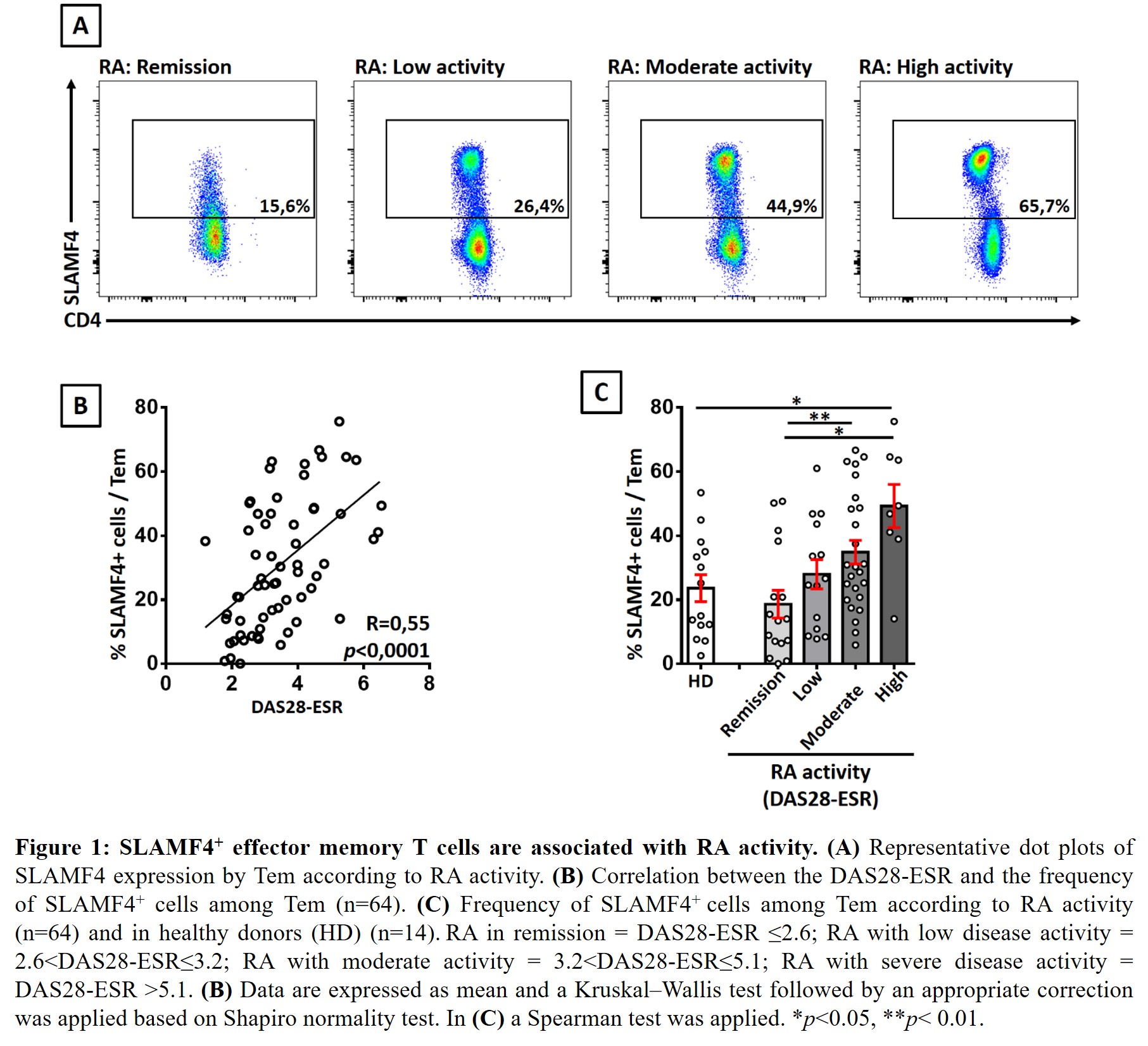
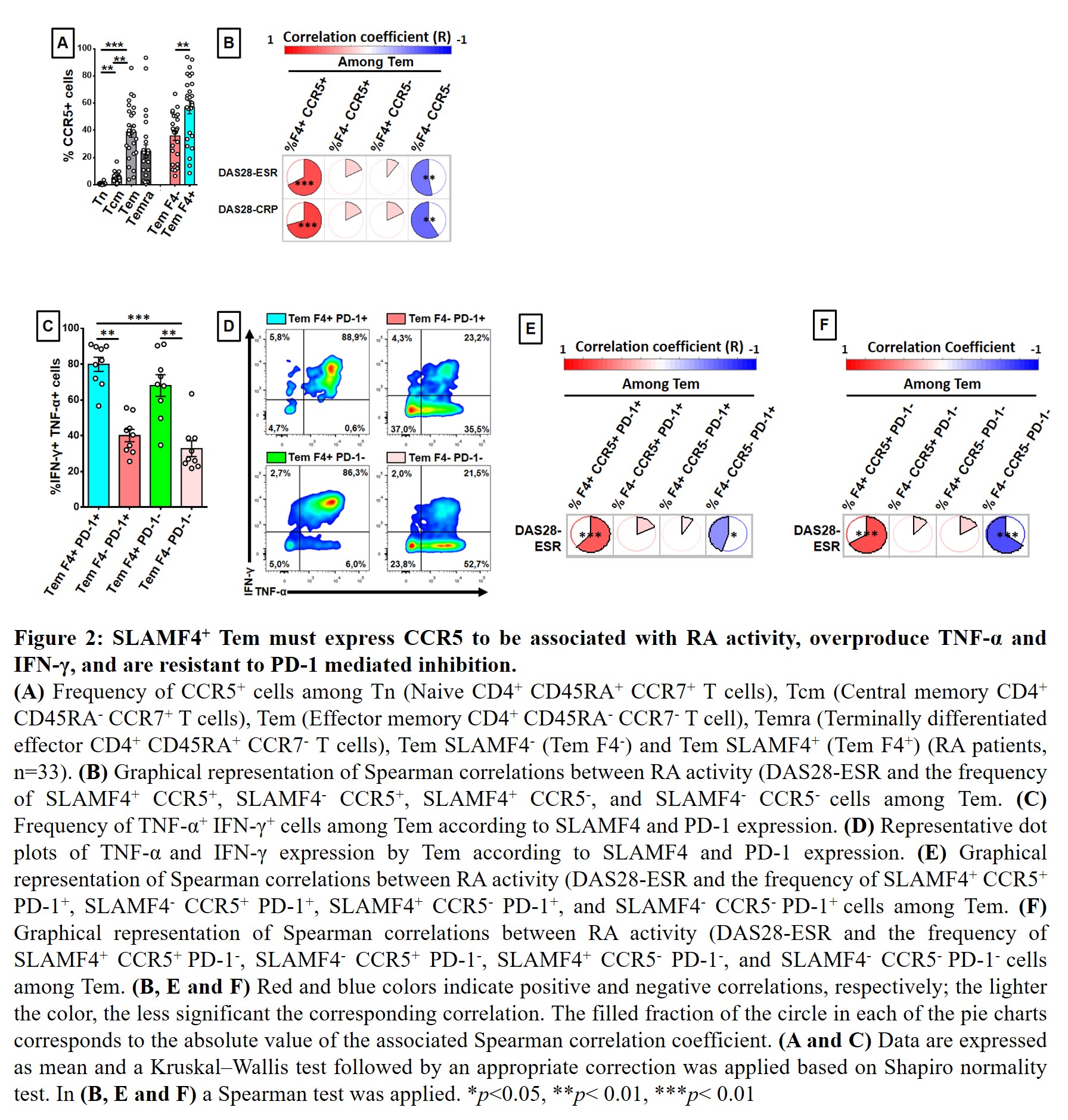
Results: Regarding the different Tconv subpopulations and SLAMFs studied, only the frequency of SLAMF4+ cells among effector memory T cells (Tem; CCR7-, CD45RA-) was correlated with disease activity (Figure 1A-B). Therefore, the frequency of SLAMF4+ cells among Tem was higher in patients with high disease activity compared with HD and patients with moderate or low disease activity (Figure 1C). In addition, SLAMF4+ Tem were characterized by a higher expression of CCR5 compared to their SLAMF4- counterpart (Figure 2A). Figure 2B showed that only SLAMF4+ Tem expressing CCR5 were linked to RA activity (DAS28-ESR vs frequency of SLAMF4+ CCR5+ cells among Tem, R=0.65, p< 0,0001). Moreover, SLAMF4+ Tem were characterized by the strongest coproduction of TNF-α and IFN-γ whatever their expression of PD-1 (Figure 2C-D). Indeed, more than half of SLAMF4+ CCR5+ Tem expressed PD-1 without altering their association with RA activity (Figure E-F).
Conclusion: Extensive cytometric characterization revealed that SLAMF4+ CCR5+ Tem represent a distinct Tconv subpopulation with polyfunctional properties, resistant to PD-1 mediated inhibition and strongly linked to RA activity. Further studies will evaluate the involvement of SLAMF4+ CCR5+ Tem in the response to targeted therapies in RA.
Disclosures: M. Lacaud, None; H. Bouzidi, None; M. Breckler, None; D. Lemeiter, None; J. Sigaux, None; L. Semerano, None; M. Boissier, None; N. Bessis, None; J. Biton, None.
Background/Purpose: CD4+ Foxp3- conventional T cells (Tconv) play a key role in the inflammatory process involved in rheumatoid arthritis (RA). Usually, chronic stimulation of T cells promotes their exhaustion. This state of anergy is induced by the co-expression of different inhibitory receptors (PD-1, TIM-3...). However, it is becoming increasingly clear that in various autoimmune diseases, chronic Tconv stimulation does not induce a sufficient level of exhaustion to inhibit their pathological response. Overexpression of activating receptors by T cells, counterbalancing that of their inhibitory counterparts, may allow them to maintain their functions in the presence of chronic activation. Among the receptors potentially capable of upregulating the Tconv response, SLAMFs (Signaling lymphocytic activation molecule) represent a family of nine receptors. In RA, our objectives were to determine whether SLAMF receptors are involved in the establishment of the Tconv pro-inflammatory response and whether these receptors represent a protection against Tconv exhaustion.
Methods: We immunophenotyped blood T cells from RA patients (n=64) and from healthy donors (HD) (n=14) using a 13-marker flow cytometry panel. The expression of SLAMF receptors was determine among four Tconv subpopulations with different activation status (naive, central memory, effector memory and terminally differentiated effector CD4+ T cells). To assess the inflammatory tropism and functionality of different Tconv subpopulations, CCR5 expression as well as TNF-α and IFN-γ secretion were studied, respectively. Comparisons between RA patient samples and HD were performed using a Kruskal–Wallis test followed by an appropriate correction. Association between Tconv subpopulation frequency and RA activity was assessed by Spearman correlations and R software was used for multiparametric graphical representations.
Results: Regarding the different Tconv subpopulations and SLAMFs studied, only the frequency of SLAMF4+ cells among effector memory T cells (Tem; CCR7-, CD45RA-) was correlated with disease activity (Figure 1A-B). Therefore, the frequency of SLAMF4+ cells among Tem was higher in patients with high disease activity compared with HD and patients with moderate or low disease activity (Figure 1C). In addition, SLAMF4+ Tem were characterized by a higher expression of CCR5 compared to their SLAMF4- counterpart (Figure 2A). Figure 2B showed that only SLAMF4+ Tem expressing CCR5 were linked to RA activity (DAS28-ESR vs frequency of SLAMF4+ CCR5+ cells among Tem, R=0.65, p< 0,0001). Moreover, SLAMF4+ Tem were characterized by the strongest coproduction of TNF-α and IFN-γ whatever their expression of PD-1 (Figure 2C-D). Indeed, more than half of SLAMF4+ CCR5+ Tem expressed PD-1 without altering their association with RA activity (Figure E-F).
Conclusion: Extensive cytometric characterization revealed that SLAMF4+ CCR5+ Tem represent a distinct Tconv subpopulation with polyfunctional properties, resistant to PD-1 mediated inhibition and strongly linked to RA activity. Further studies will evaluate the involvement of SLAMF4+ CCR5+ Tem in the response to targeted therapies in RA.


Disclosures: M. Lacaud, None; H. Bouzidi, None; M. Breckler, None; D. Lemeiter, None; J. Sigaux, None; L. Semerano, None; M. Boissier, None; N. Bessis, None; J. Biton, None.

