Back
Poster Session B
Rheumatoid arthritis (RA)
Session: (0589–0628) RA – Etiology and Pathogenesis Poster
0624: CD20+ T Cells in the Synovial Fluid of Patients with Rheumatoid Arthritis Suggest a Role for Trogocytosis in Disease Pathogenesis and Activity
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- MD
Maria De Santis, MD, PhD
Humanitas University
Pieve Emanuele, Milan, Italy
Abstract Poster Presenter(s)
Maria De Santis1, Natasa Isailovic2, Giacomo Maria Guidelli3, Daniela Renna4, Arianna Sonaglia3, Nicoletta Luciano3, Marta Caprioli5, Angela Ceribelli1, Francesca Motta6, Matteo Vecellio1, Enrico Brunetta3 and Carlo Selmi1, 1Humanitas University, IRCCS Humanitas Research Hospital, Rheumatology and Clinical Immunology, Pieve Emanuele, Italy, 2IRCCS Humanitas Research Hospital, Rozzano (MI), Italy, Rozzano, Italy, 3IRCCS Humanitas Research Hospital, Rheumatology and Clinical Immunology, Rozzano, Italy, 4IRCCS Humanitas Research Hospital, Rheumatology and Clinical Immunology, Milano, Italy, 5IRCCS Humanitas Research Hospital, Rheumatology and Clinical Immunology, Pavia, Italy, 6Humanitas University, IRCCS Humanitas Research Hospital, Rheumatology and Clinical Immunology, Rozzano, Italy
Background/Purpose: Approximately 1-2% of peripheral blood T cells express CD20, a marker of the B lineage, in both healthy individuals and patients with rheumatoid arthritis (RA). This may be secondary to trogocytosis, that is the active process in which one cell extracts the cell fragment, including surface molecules, from another cell. The immunological significance of this population is not clear but in RA CD20+ T cells are highly activated and constitutively produce proinflammatory cytokines. Of note, CD20-targeting therapies, i.e. rituximab, lead to the depletion of CD20+T cells along with B cells but the contribution, as an additional mechanism, to the efficacy of rituximab in patients with RA has been hypothesized (Wilk E, et al. Arthritis Rheum 2009). A pathogenic role of CD20+ T cells has been found in multiple sclerosis animal model: the adoptive transfer of CD20+ T cells exacerbates clinical and histological experimental model of multiple sclerosis; moreover, anti-CD20–mediated depletion of CD20+ T cells was therapeutically beneficial independently of B cells in patients with multiple sclerosis (Ochs J, et al. Science Transl Med 2022).
Methods: Synovial fluid samples were obtained from 4 patients with established RA with knee involvement [2 females; median age 67, (interquartile range - IQR 45-74)]. All the patients were seropositive for rheumatoid factors and anticitrullinated protein antibodies and fulfilled the ACR criteria for RA. One patient was not on therapy, 1 patient on Salazopyrin 2g/day, 1 on Salazopyrin 2 g/day and Abatacept/weekly, 1 on methotrexate and anti-TNFα biologic; Disease activity was measured by DAS28 and scored median 4.15 (IQR 3.04-6.17). Samples were investigated for the presence of CD20+ T lymphocytes by FACS along with the expression of CD19, CD4 and CD8.
Results: Synovial fluid B cells (live lymphocyte gate, CD19+) were 0.1-3.1(min-max)% with 56-86% of cells coexpressing also CD20. Synovial fluid T cells (live lymphocyte gate, CD3+) were 57.8-89.6% with 7.7-20% coexpressing CD20 (Figure 1). Among CD20+ T cells, 60.7-65.4% coexpressed CD8 and 24.2-35.6% coexpressed CD4 (Figure 1). Mean fluorescence intensity (MFI) of CD20 was 19407-23430 (min-max) in B cells compared to 1152-1370 in T cells. A positive correlation was found between MFI of CD20 in T cells and DAS28 (Spearman’s rho coefficient 0.8).
Conclusion: The analysis of a small number of patients with RA demonstrates that T cells expressing CD20 are found in the synovial fluid and the level of CD20 per cell correlates with disease activity. Our data suggest that trogocytosis leading to CD20 expression on synovial fluid T cells may be involved in the disease pathogenesis and possibly contribute to the response to rituximab.
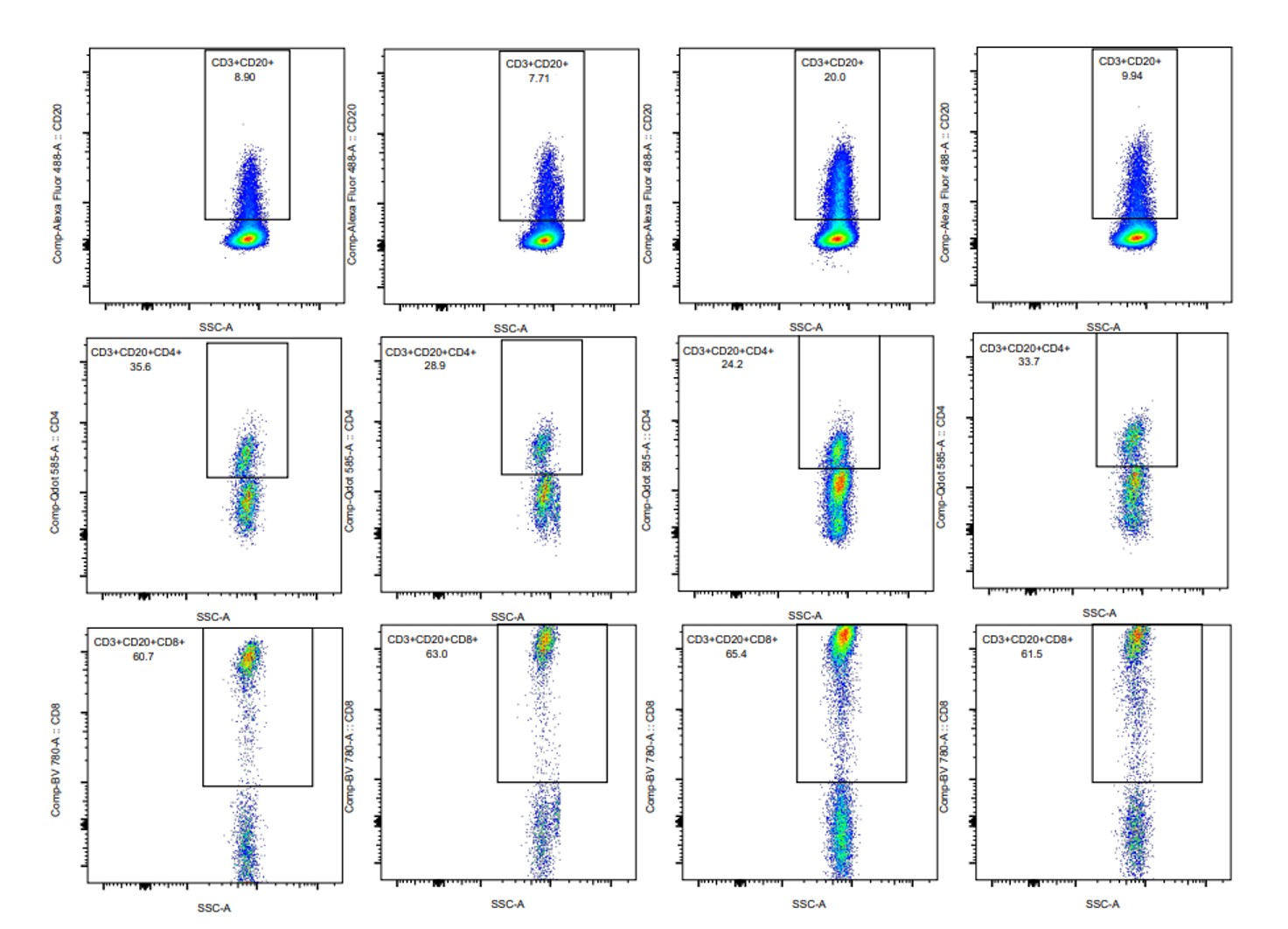 Percentages of synovial fluid CD20+ T cells and CD4 or CD8 subsets in 4 patients suffering from rheumatoid arthritis
Percentages of synovial fluid CD20+ T cells and CD4 or CD8 subsets in 4 patients suffering from rheumatoid arthritis
Disclosures: M. De Santis, None; N. Isailovic, None; G. Guidelli, None; D. Renna, None; A. Sonaglia, None; N. Luciano, None; M. Caprioli, None; A. Ceribelli, None; F. Motta, None; M. Vecellio, None; E. Brunetta, None; C. Selmi, AbbVie, Amgen, Pfizer, Alfa-Wasserman, Biogen, Eli Lilly, Galapagos, Janssen, Novartis, SOBI.
Background/Purpose: Approximately 1-2% of peripheral blood T cells express CD20, a marker of the B lineage, in both healthy individuals and patients with rheumatoid arthritis (RA). This may be secondary to trogocytosis, that is the active process in which one cell extracts the cell fragment, including surface molecules, from another cell. The immunological significance of this population is not clear but in RA CD20+ T cells are highly activated and constitutively produce proinflammatory cytokines. Of note, CD20-targeting therapies, i.e. rituximab, lead to the depletion of CD20+T cells along with B cells but the contribution, as an additional mechanism, to the efficacy of rituximab in patients with RA has been hypothesized (Wilk E, et al. Arthritis Rheum 2009). A pathogenic role of CD20+ T cells has been found in multiple sclerosis animal model: the adoptive transfer of CD20+ T cells exacerbates clinical and histological experimental model of multiple sclerosis; moreover, anti-CD20–mediated depletion of CD20+ T cells was therapeutically beneficial independently of B cells in patients with multiple sclerosis (Ochs J, et al. Science Transl Med 2022).
Methods: Synovial fluid samples were obtained from 4 patients with established RA with knee involvement [2 females; median age 67, (interquartile range - IQR 45-74)]. All the patients were seropositive for rheumatoid factors and anticitrullinated protein antibodies and fulfilled the ACR criteria for RA. One patient was not on therapy, 1 patient on Salazopyrin 2g/day, 1 on Salazopyrin 2 g/day and Abatacept/weekly, 1 on methotrexate and anti-TNFα biologic; Disease activity was measured by DAS28 and scored median 4.15 (IQR 3.04-6.17). Samples were investigated for the presence of CD20+ T lymphocytes by FACS along with the expression of CD19, CD4 and CD8.
Results: Synovial fluid B cells (live lymphocyte gate, CD19+) were 0.1-3.1(min-max)% with 56-86% of cells coexpressing also CD20. Synovial fluid T cells (live lymphocyte gate, CD3+) were 57.8-89.6% with 7.7-20% coexpressing CD20 (Figure 1). Among CD20+ T cells, 60.7-65.4% coexpressed CD8 and 24.2-35.6% coexpressed CD4 (Figure 1). Mean fluorescence intensity (MFI) of CD20 was 19407-23430 (min-max) in B cells compared to 1152-1370 in T cells. A positive correlation was found between MFI of CD20 in T cells and DAS28 (Spearman’s rho coefficient 0.8).
Conclusion: The analysis of a small number of patients with RA demonstrates that T cells expressing CD20 are found in the synovial fluid and the level of CD20 per cell correlates with disease activity. Our data suggest that trogocytosis leading to CD20 expression on synovial fluid T cells may be involved in the disease pathogenesis and possibly contribute to the response to rituximab.
 Percentages of synovial fluid CD20+ T cells and CD4 or CD8 subsets in 4 patients suffering from rheumatoid arthritis
Percentages of synovial fluid CD20+ T cells and CD4 or CD8 subsets in 4 patients suffering from rheumatoid arthritisDisclosures: M. De Santis, None; N. Isailovic, None; G. Guidelli, None; D. Renna, None; A. Sonaglia, None; N. Luciano, None; M. Caprioli, None; A. Ceribelli, None; F. Motta, None; M. Vecellio, None; E. Brunetta, None; C. Selmi, AbbVie, Amgen, Pfizer, Alfa-Wasserman, Biogen, Eli Lilly, Galapagos, Janssen, Novartis, SOBI.

