Back
Poster Session D
Sjögren's syndrome
Session: (2017–2051) Sjögren's Syndrome – Basic and Clinical Science Poster
2050: Stratification on Baseline Interferon Scores and Use of CRESS to Improve the Design of Future Trials in Sjögren's Syndrome: Post Hoc Analysis from a Randomized Controlled Trial
Monday, November 14, 2022
1:00 PM – 3:00 PM Eastern Time
Location: Virtual Poster Hall
- JG
Jacques-Eric Gottenberg, PhD
CHU
Strasbourg, France
Abstract Poster Presenter(s)
jacques-eric gottenberg1, Bryan Downie2, Oksana Gurtovaya2 and Anubhav Mathur2, 1Strasbourg University Hospital, Strasbourg, France, 2Gilead Sciences, Foster City, CA
Background/Purpose: A multicenter, global, randomized, Phase 2, double-blind, placebo (PBO)-controlled study showed filgotinib (FIL; 200 mg/d), lanraplenib (LAN; 30 mg/d), and tirabrutinib (TIRA; 40 mg/d) were well tolerated by patients (pts) with Sjögren’s syndrome (SS; EULAR SS disease activity index [ESSDAI] score ≥5).1 However, the primary endpoint (a composite based on high-sensitivity C-reactive protein response at Week [W]12) was not met, possibly due to endpoint choice and inclusion of primary and associated SS. Here, we investigate biomarkers predictive of treatment response and possible relationships between baseline (BL) interferon (IFN) activity and clinical response rates to FIL, TIRA, and LAN using the novel Composite of Relevant Endpoints for Sjögren’s Syndrome (CRESS)2 score as a clinical endpoint.
Methods: A post hoc analysis of data from NCT031009421 (Fig 1) of 150 adults with primary or associated SS was conducted. Primary SS was characterized by the absence of concurrent autoimmune connective tissue diseases, while having such comorbidities was deemed associated SS. Overall, 302 peripheral biomarkers were assessed at BL and throughout the study, including whole-blood gene expression profiling, where a BL IFN signature score was calculated.1 Pts were classified as IFN-high or IFN-low based on two standard deviations above the healthy volunteer mean. CRESS response was defined as ≥3 of the 5 CRESS items (systemic disease activity, patient-reported index, tear gland item, salivary gland item, and serology).2 Comparisons to PBO were assessed using a Fisher’s-exact test, without multiplicity adjustment.
Results: There were no biomarker or transcriptional pathway differences distinguishing primary from associated SS. Thus, data were pooled for subsequent analyses. CRESS response was similar for LAN vs PBO, while numerically higher rates were observed for FIL and TIRA vs PBO at W12 and W24 (Fig 2).
Among pts with IFN-low scores, no significant differences were observed in CRESS responses across treatment arms (Fig 3). In contrast, among pts with IFN-high scores, CRESS responses to FIL at W12 (+33%, p = 0.022) and W24 (Fig 3; +40%, p = 0.015) were significantly higher compared to PBO in pts with BL IFN-high scores. CRESS response rates to TIRA were significantly greater than PBO at W12 (+28%, p = 0.038) and numerically but not significantly greater than PBO at W24 (Fig 3; +17%, p = 0.25).
Conclusion: This post hoc analysis showed no biomarkers or transcriptional pathways that distinguished primary from associated SS. Thus, there is no clear scientific reason to restrict inclusion criteria to primary SS in clinical trials. Our analysis also indicates that BL IFN activity correlated with CRESS response to FIL (a Janus kinase-1 inhibitor) and TIRA (a Bruton’s tyrosine kinase inhibitor), despite distinct mechanisms of action. This could suggest a role for stratifying SS pts by BL IFN score and reinforces the rationale of using novel composite endpoints, like CRESS in future trials.
References
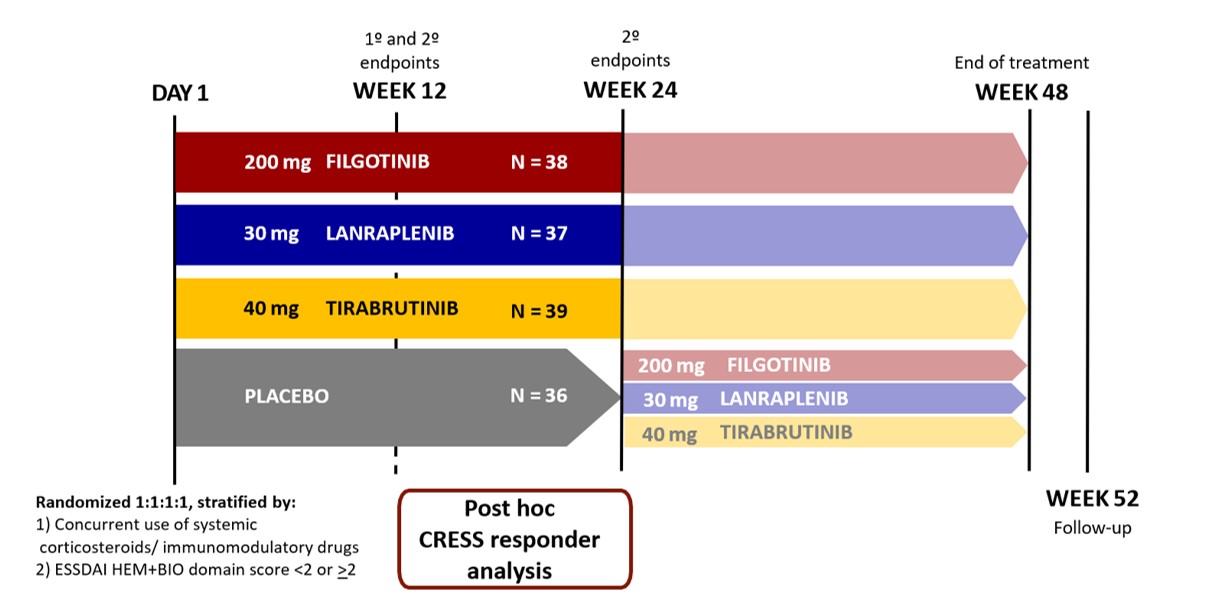 Figure 1. Study design.
Figure 1. Study design.
Adapted from Price et al. 2022.
BIO, biological component score; CRESS, Composite of Relevant Endpoints for Sjögren’s Syndrome; ESSDAI, EULAR Sjögren’s syndrome disease activity index; HEM, hematologic component score.
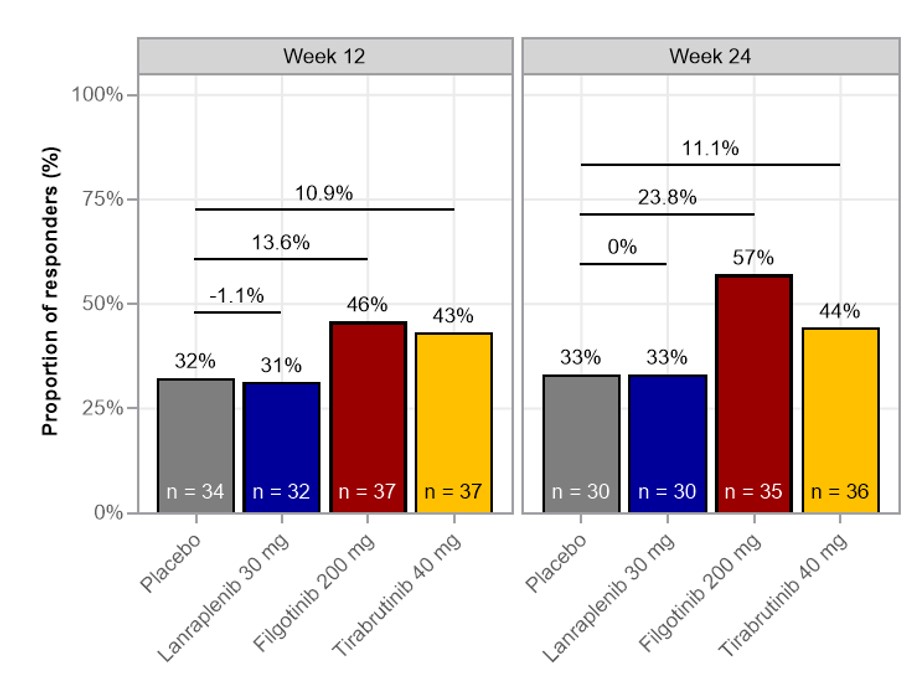 Figure 2. Proportion of CRESS responders in the full analysis set .
Figure 2. Proportion of CRESS responders in the full analysis set .
CRESS, Composite of Relevant Endpoints for Sjögren’s Syndrome.
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1288134-3-ANY.jpg width=440 height=273.277661795407 border=0 style=border-style: none;>
Disclosures: j. gottenberg, AbbVie, Bristol Myers Squibb, Galapagos, Gilead, Lilly, MSD, Novartis, Pfizer; B. Downie, Gilead; O. Gurtovaya, Gilead Sciences; A. Mathur, Gilead.
Background/Purpose: A multicenter, global, randomized, Phase 2, double-blind, placebo (PBO)-controlled study showed filgotinib (FIL; 200 mg/d), lanraplenib (LAN; 30 mg/d), and tirabrutinib (TIRA; 40 mg/d) were well tolerated by patients (pts) with Sjögren’s syndrome (SS; EULAR SS disease activity index [ESSDAI] score ≥5).1 However, the primary endpoint (a composite based on high-sensitivity C-reactive protein response at Week [W]12) was not met, possibly due to endpoint choice and inclusion of primary and associated SS. Here, we investigate biomarkers predictive of treatment response and possible relationships between baseline (BL) interferon (IFN) activity and clinical response rates to FIL, TIRA, and LAN using the novel Composite of Relevant Endpoints for Sjögren’s Syndrome (CRESS)2 score as a clinical endpoint.
Methods: A post hoc analysis of data from NCT031009421 (Fig 1) of 150 adults with primary or associated SS was conducted. Primary SS was characterized by the absence of concurrent autoimmune connective tissue diseases, while having such comorbidities was deemed associated SS. Overall, 302 peripheral biomarkers were assessed at BL and throughout the study, including whole-blood gene expression profiling, where a BL IFN signature score was calculated.1 Pts were classified as IFN-high or IFN-low based on two standard deviations above the healthy volunteer mean. CRESS response was defined as ≥3 of the 5 CRESS items (systemic disease activity, patient-reported index, tear gland item, salivary gland item, and serology).2 Comparisons to PBO were assessed using a Fisher’s-exact test, without multiplicity adjustment.
Results: There were no biomarker or transcriptional pathway differences distinguishing primary from associated SS. Thus, data were pooled for subsequent analyses. CRESS response was similar for LAN vs PBO, while numerically higher rates were observed for FIL and TIRA vs PBO at W12 and W24 (Fig 2).
Among pts with IFN-low scores, no significant differences were observed in CRESS responses across treatment arms (Fig 3). In contrast, among pts with IFN-high scores, CRESS responses to FIL at W12 (+33%, p = 0.022) and W24 (Fig 3; +40%, p = 0.015) were significantly higher compared to PBO in pts with BL IFN-high scores. CRESS response rates to TIRA were significantly greater than PBO at W12 (+28%, p = 0.038) and numerically but not significantly greater than PBO at W24 (Fig 3; +17%, p = 0.25).
Conclusion: This post hoc analysis showed no biomarkers or transcriptional pathways that distinguished primary from associated SS. Thus, there is no clear scientific reason to restrict inclusion criteria to primary SS in clinical trials. Our analysis also indicates that BL IFN activity correlated with CRESS response to FIL (a Janus kinase-1 inhibitor) and TIRA (a Bruton’s tyrosine kinase inhibitor), despite distinct mechanisms of action. This could suggest a role for stratifying SS pts by BL IFN score and reinforces the rationale of using novel composite endpoints, like CRESS in future trials.
References
1. Price E et al. Rheumatology. OBP April 4, 2022. DOI: 10.1093/rheumatology/keac167. 2. Arends S et al. Lancet Rheumatol. 2021;3:553-62.
 Figure 1. Study design.
Figure 1. Study design.Adapted from Price et al. 2022.
BIO, biological component score; CRESS, Composite of Relevant Endpoints for Sjögren’s Syndrome; ESSDAI, EULAR Sjögren’s syndrome disease activity index; HEM, hematologic component score.
 Figure 2. Proportion of CRESS responders in the full analysis set .
Figure 2. Proportion of CRESS responders in the full analysis set .CRESS, Composite of Relevant Endpoints for Sjögren’s Syndrome.
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/17574/QHOPTGBB-1288134-3-ANY.jpg width=440 height=273.277661795407 border=0 style=border-style: none;>
Figure 3. Week 24 CRESS response rate by IFN status at baseline.
*p < 0.05. IFN-High >2 SD above the healthy volunteer mean.
CRESS, Composite of Relevant Endpoints for Sjögren’s Syndrome; IFN, interferon.
*p < 0.05. IFN-High >2 SD above the healthy volunteer mean.
CRESS, Composite of Relevant Endpoints for Sjögren’s Syndrome; IFN, interferon.
Disclosures: j. gottenberg, AbbVie, Bristol Myers Squibb, Galapagos, Gilead, Lilly, MSD, Novartis, Pfizer; B. Downie, Gilead; O. Gurtovaya, Gilead Sciences; A. Mathur, Gilead.

