Back
Poster Session B
Antiphospholipid Syndrome
Session: (0671–0694) Antiphospholipid Syndrome Poster
0675: Clinical Characteristics and Factors Associated with Relapse and Mortality in Diffuse Alveolar Hemorrhage Among Patients with Antiphospholipid Syndrome: A Multi-Center Retrospective Cohort
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- JM
Jose Meade-Aguilar, MD
Mayo Clinic
Rochester, MN, United States
Abstract Poster Presenter(s)
Jose A Meade-Aguilar1, Gabriel Figueroa Parra1, Hannah Langenfeld2, Rodrigo Cartin-Ceba1, Ulrich Specks1, Cynthia Crowson3 and Ali Duarte-Garcia1, 1Mayo Clinic, Rochester, MN, 2Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN, 3Mayo Clinic, Eyota, MN
Background/Purpose: Antiphospholipid syndrome (APS) is associated with diffuse alveolar hemorrhage (DAH). However, only case reports and small case series are available in the literature. We aimed to describe the clinical characteristics, therapeutic strategies, and clinical outcomes of patients with APS and DAH from a multi-center institution.
Methods:
We conducted a multi-center, retrospective, cohort study of patients with incident APS-associated DAH from a healthcare system with 19 hospitals and affiliated outpatient centers in 4 states across the US. Patients meeting the APS Sydney criteria were included. DAH was defined as the presence of suggestive radiographic findings and a progressively bloody bronchoalveolar lavage (BAL) fluid and/or >20% hemosiderin-laden macrophages in the BAL or biopsy with evidence of DAH and capillaritis. Patients with DAH explained by another condition were excluded. We abstracted demographics, SLE diagnosis, APS manifestations, symptoms, laboratory, radiologic, BAL findings, and treatments at DAH episode; outcomes (relapse and mortality) were abstracted during follow-up. Patients were followed until death or last visit withing the health system. Descriptive statistics were used to summarize data, Kaplan-Meier methods were used to estimate relapse and mortality rates, and Cox models were used to evaluate factors associated with relapse and mortality.
Results: We included 61 patients with APS and incident DAH. The mean age was 44.2 (SD 15.93) years, 48% were female, 87% were non-Hispanic White, and 30% had SLE. The median APS duration was 4.4 years, and the median follow-up was 32.3 months. 85% of patients had a history of thromboembolism, 36% stroke, 28% myocardial infarction, and 17% of women had a history of pregnancy morbidity; the most common “non-criteria” manifestations were thrombocytopenia (51%), and valvulopathy (43%). At DAH onset, 90% of patients had dyspnea, 79% cough, and 59% hemoptysis; most of the cases had ground glass opacities or consolidations on CT scan or radiographs (87% and 90%, respectively), while 90% had a positive BAL (Table 1).Before DAH, 74% of the patients were anticoagulated. Among the therapeutic strategies used, 48% received pulses of corticosteroids, 23% MMF, 20% Rituximab, 13% CYC, and 20% plasma exchange. The relapse rate was 41.2% at 1-year, 16 patients (26%) relapsed within six months (Figure 1A). Factors associated with relapse within six months were triple positivity (HR 8.50), thrombocytopenia < 50K/µL (HR 5.26), IVIG (HR 5.95), plasma exchange use (HR 3.35), and prior mechanical ventilation (HR 4.26). The estimated mortality at 1 and 5 years was 19.2% and 38.2% (Figure 1B). Factors associated with mortality were thrombocytopenia < 50K/µL (HR 5.88) and age (HR 1.44 per 10 years) (Table 2).
Conclusion: In the largest cohort of patients with incident APS-associated DAH, we found a high mortality, and a significant relapse risk within the first six months. A positive aCL IgG, triple positivity, and thrombocytopenia were risk factors for relapses, while thrombocytopenia was also associated with mortality.
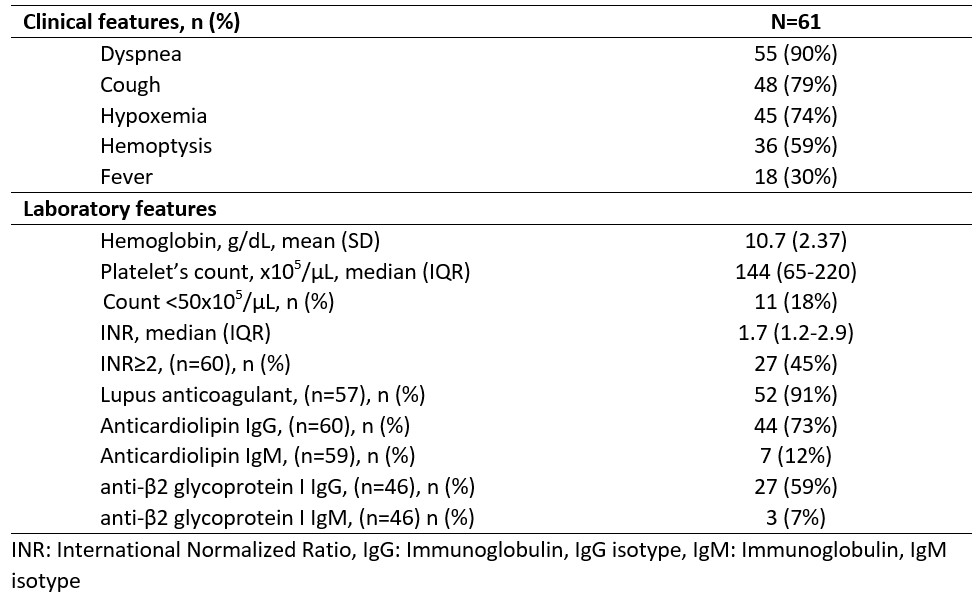 Table 1.Frequency of clinical and laboratory features, and antiphospholipid antibodies at presentation of diffuse alveolar hemorrhage episodes among patients with APS.
Table 1.Frequency of clinical and laboratory features, and antiphospholipid antibodies at presentation of diffuse alveolar hemorrhage episodes among patients with APS.
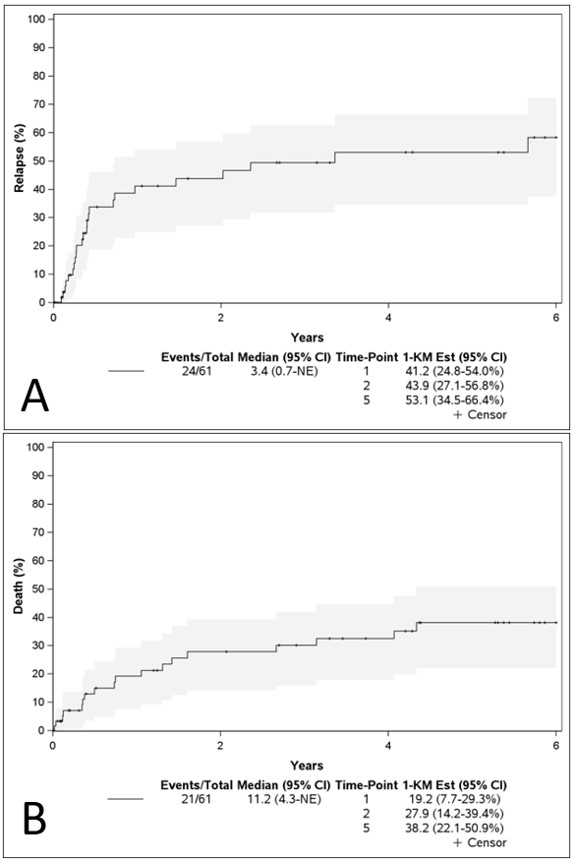 Figure 1. Cumulative incidence curves for A) relapse and B) mortality of patients with incident diffuse alveolar hemorrhage and antiphospholipid syndrome.
Figure 1. Cumulative incidence curves for A) relapse and B) mortality of patients with incident diffuse alveolar hemorrhage and antiphospholipid syndrome.
.jpg) Table 2. Cox model adjusted for age and sex for relapse and mortality in patients with diffuse alveolar hemorrhage episodes and antiphospholipid syndrome.
Table 2. Cox model adjusted for age and sex for relapse and mortality in patients with diffuse alveolar hemorrhage episodes and antiphospholipid syndrome.
Disclosures: J. Meade-Aguilar, None; G. Figueroa Parra, None; H. Langenfeld, None; R. Cartin-Ceba, None; U. Specks, AstraZeneca, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Genentech, AstraZeneca, Boehringer-Ingelheim, ChemoCentryx; C. Crowson, None; A. Duarte-Garcia, None.
Background/Purpose: Antiphospholipid syndrome (APS) is associated with diffuse alveolar hemorrhage (DAH). However, only case reports and small case series are available in the literature. We aimed to describe the clinical characteristics, therapeutic strategies, and clinical outcomes of patients with APS and DAH from a multi-center institution.
Methods:
We conducted a multi-center, retrospective, cohort study of patients with incident APS-associated DAH from a healthcare system with 19 hospitals and affiliated outpatient centers in 4 states across the US. Patients meeting the APS Sydney criteria were included. DAH was defined as the presence of suggestive radiographic findings and a progressively bloody bronchoalveolar lavage (BAL) fluid and/or >20% hemosiderin-laden macrophages in the BAL or biopsy with evidence of DAH and capillaritis. Patients with DAH explained by another condition were excluded. We abstracted demographics, SLE diagnosis, APS manifestations, symptoms, laboratory, radiologic, BAL findings, and treatments at DAH episode; outcomes (relapse and mortality) were abstracted during follow-up. Patients were followed until death or last visit withing the health system. Descriptive statistics were used to summarize data, Kaplan-Meier methods were used to estimate relapse and mortality rates, and Cox models were used to evaluate factors associated with relapse and mortality.
Results: We included 61 patients with APS and incident DAH. The mean age was 44.2 (SD 15.93) years, 48% were female, 87% were non-Hispanic White, and 30% had SLE. The median APS duration was 4.4 years, and the median follow-up was 32.3 months. 85% of patients had a history of thromboembolism, 36% stroke, 28% myocardial infarction, and 17% of women had a history of pregnancy morbidity; the most common “non-criteria” manifestations were thrombocytopenia (51%), and valvulopathy (43%). At DAH onset, 90% of patients had dyspnea, 79% cough, and 59% hemoptysis; most of the cases had ground glass opacities or consolidations on CT scan or radiographs (87% and 90%, respectively), while 90% had a positive BAL (Table 1).Before DAH, 74% of the patients were anticoagulated. Among the therapeutic strategies used, 48% received pulses of corticosteroids, 23% MMF, 20% Rituximab, 13% CYC, and 20% plasma exchange. The relapse rate was 41.2% at 1-year, 16 patients (26%) relapsed within six months (Figure 1A). Factors associated with relapse within six months were triple positivity (HR 8.50), thrombocytopenia < 50K/µL (HR 5.26), IVIG (HR 5.95), plasma exchange use (HR 3.35), and prior mechanical ventilation (HR 4.26). The estimated mortality at 1 and 5 years was 19.2% and 38.2% (Figure 1B). Factors associated with mortality were thrombocytopenia < 50K/µL (HR 5.88) and age (HR 1.44 per 10 years) (Table 2).
Conclusion: In the largest cohort of patients with incident APS-associated DAH, we found a high mortality, and a significant relapse risk within the first six months. A positive aCL IgG, triple positivity, and thrombocytopenia were risk factors for relapses, while thrombocytopenia was also associated with mortality.
 Table 1.Frequency of clinical and laboratory features, and antiphospholipid antibodies at presentation of diffuse alveolar hemorrhage episodes among patients with APS.
Table 1.Frequency of clinical and laboratory features, and antiphospholipid antibodies at presentation of diffuse alveolar hemorrhage episodes among patients with APS. Figure 1. Cumulative incidence curves for A) relapse and B) mortality of patients with incident diffuse alveolar hemorrhage and antiphospholipid syndrome.
Figure 1. Cumulative incidence curves for A) relapse and B) mortality of patients with incident diffuse alveolar hemorrhage and antiphospholipid syndrome..jpg) Table 2. Cox model adjusted for age and sex for relapse and mortality in patients with diffuse alveolar hemorrhage episodes and antiphospholipid syndrome.
Table 2. Cox model adjusted for age and sex for relapse and mortality in patients with diffuse alveolar hemorrhage episodes and antiphospholipid syndrome. Disclosures: J. Meade-Aguilar, None; G. Figueroa Parra, None; H. Langenfeld, None; R. Cartin-Ceba, None; U. Specks, AstraZeneca, Bristol-Myers Squibb(BMS), GlaxoSmithKlein(GSK), Genentech, AstraZeneca, Boehringer-Ingelheim, ChemoCentryx; C. Crowson, None; A. Duarte-Garcia, None.

