Back
Poster Session B
Systemic lupus erythematosus (SLE)
Session: (0629–0670) SLE – Etiology and Pathogenesis Poster
0659: Comprehensive Proteomic Screen of Murine Lupus Serum and Cerebrospinal Fluid Uncovers Diagnostic and Therapeutic Targets
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- JR
Joshua Reynolds, MS, BS
Albert Einstein COM
Bronx, NY, United States
Abstract Poster Presenter(s)
Joshua Reynolds1, Chandra Mohan2, Yaxi Li2 and Chaim Putterman1, 1Albert Einstein College of Medicine, Bronx, NY, 2University of Houston, Houston, TX
Background/Purpose: Up to 50% of SLE patients experience neuropsychiatric involvement (neuropsychiatric lupus, or NPLSE) through an unknown mechanism. Diagnosis currently relies on clinical criteria and physician experience; novel biomarkers are needed to identify patients more rapidly and reliably. Studying the central nervous system (CNS) pathology in the classic MRL/lpr mouse model has been hindered by the lack of cerebrospinal fluid (CSF) characterization; how this fluid might link systemic disease and neuropathology is unknown. We aimed to identify protein biomarkers of NPSLE-like disease, comprehensively characterize the proteome of the MRL/lpr mouse CSF, and better understand the mechanism of NPSLE.
Methods: The MRL/lpr mouse (lpr) strain exhibits significantly worse clinical disease in females and neuropsychiatric deficits at an early age. Serum from female MRL/lpr (age 17-18 weeks; n = 8) and control MRL/mpj (mpj; age 17-18 weeks; n = 8) was collected. CSF from 3 cohorts of lpr (age 14-18 weeks; n = 10-15 per cohort) and 3 cohorts of mpj (age 14-18 weeks; n = 10-15 per cohort) was collected and pooled into six, 100uL samples (lpr n = 3; mpj n = 3). A validated behavioral testing battery assessed murine correlates of neuropsychiatric disease. RayBiotech mouse protein arrays were used to detect levels of 1308 proteins in serum and CSF. Fluorescence intensities from arrays were normalized to internal standards and controls. Fold change (FC) and multiple-test-corrected Mann Whitney p-values determined viable biomarkers (serum: FC > 1.5 or < 0.6 and p < 0.05; CSF: only p < 0.1). Pearson correlation identified serum proteins related to lpr behavioral testing scores (p < 0.05). DAVID software identified top gene ontology terms and KEGG pathways corresponding to biomarkers.
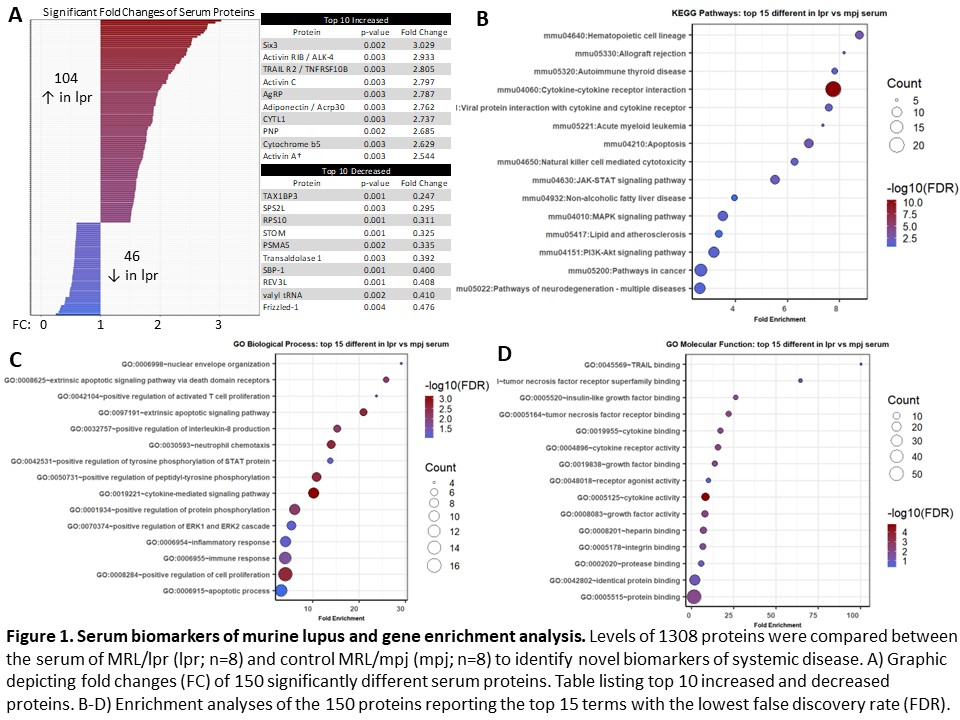
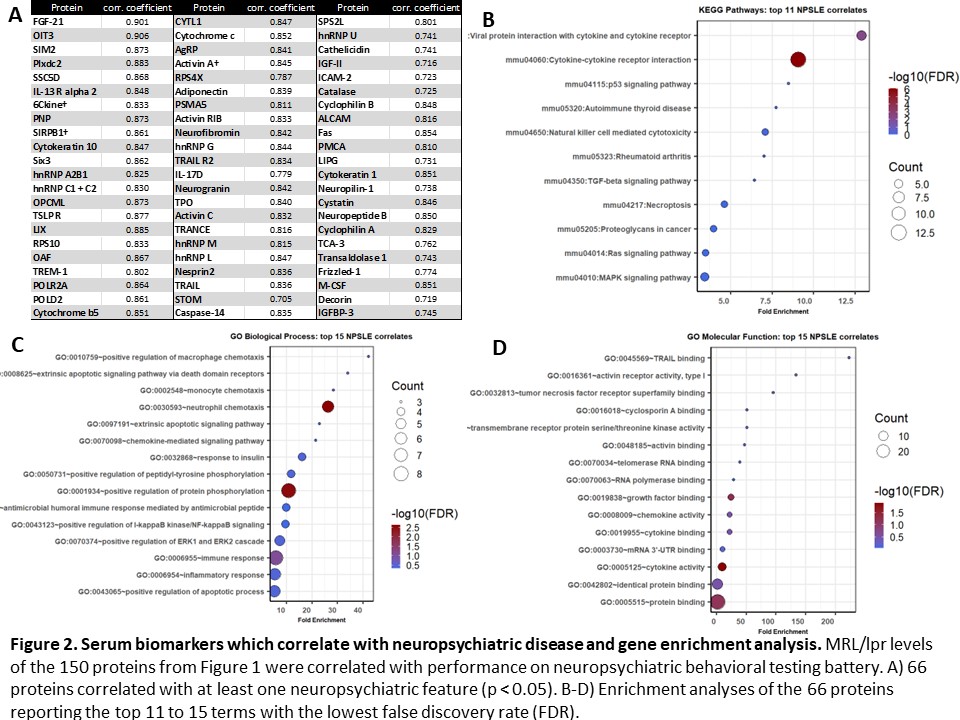
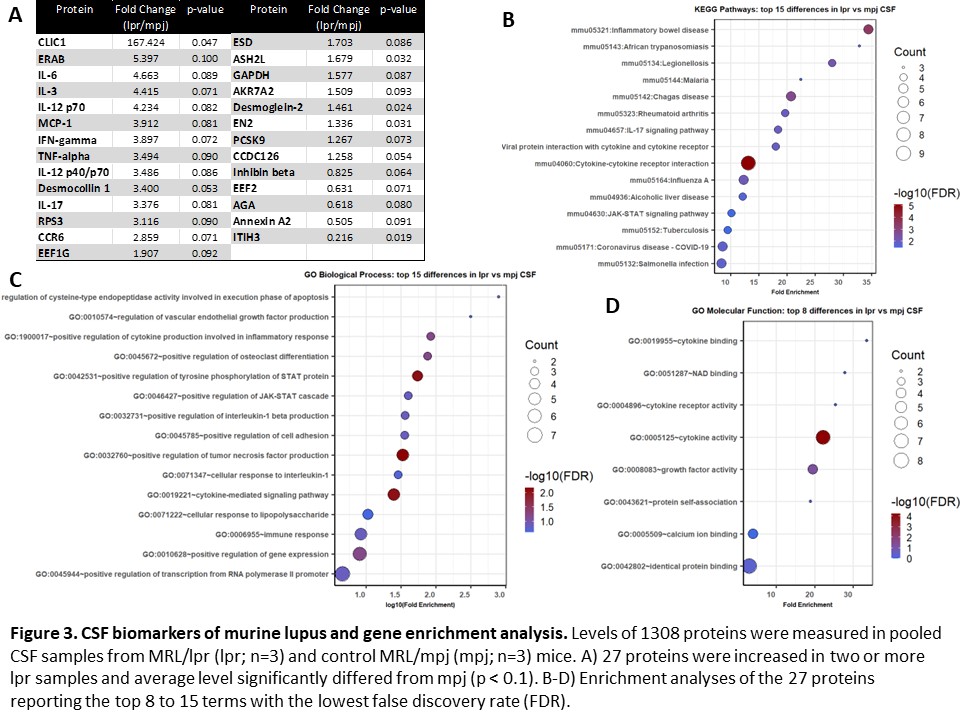
Results: 150 serum proteins distinguished between lupus mice and non-lupus controls (p < 0.05). Of these, 104 were increased and 46 were decreased in lupus mice (Figure 1A). Significant enrichment in cytokine signaling, apoptotic activity, and cellular immune responses was present (Figure 1B-D). Among those 150 proteins, 66 correlated with at least one feature of murine NPSLE (Figure 2A). From this subset, enrichment analyses showed involvement of cytokine signaling and innate immunity (Figure 2B-D). 27 CSF proteins distinguished between lupus mice and controls (increased in two or more lpr samples; p < 0.1), including several inflammatory cytokines and neuronal products (Figure 3A). Enrichment analyses identified inflammatory disease activity and cytokine signaling in the central nervous system (Figure 3B-D).
Conclusion: We succeeded in identifying novel serum biomarkers of murine NPSLE; several chemokines, cytokines, and neuronal proteins could facilitate systemic immune system targeting of the CNS. For the first time, a comprehensive picture of the MRL/lpr mouse CSF has been produced. Several inflammatory and neurotoxic cytokines are increased, indicating significant neuroinflammatory activity in lupus. In addition to finding possible diagnostic markers of NPSLE, these results also help clarify its mechanism and identify putative therapeutic targets.
Disclosures: J. Reynolds, None; C. Mohan, None; Y. Li, None; C. Putterman, Equillium, Progentec, Kidneycure.
Background/Purpose: Up to 50% of SLE patients experience neuropsychiatric involvement (neuropsychiatric lupus, or NPLSE) through an unknown mechanism. Diagnosis currently relies on clinical criteria and physician experience; novel biomarkers are needed to identify patients more rapidly and reliably. Studying the central nervous system (CNS) pathology in the classic MRL/lpr mouse model has been hindered by the lack of cerebrospinal fluid (CSF) characterization; how this fluid might link systemic disease and neuropathology is unknown. We aimed to identify protein biomarkers of NPSLE-like disease, comprehensively characterize the proteome of the MRL/lpr mouse CSF, and better understand the mechanism of NPSLE.
Methods: The MRL/lpr mouse (lpr) strain exhibits significantly worse clinical disease in females and neuropsychiatric deficits at an early age. Serum from female MRL/lpr (age 17-18 weeks; n = 8) and control MRL/mpj (mpj; age 17-18 weeks; n = 8) was collected. CSF from 3 cohorts of lpr (age 14-18 weeks; n = 10-15 per cohort) and 3 cohorts of mpj (age 14-18 weeks; n = 10-15 per cohort) was collected and pooled into six, 100uL samples (lpr n = 3; mpj n = 3). A validated behavioral testing battery assessed murine correlates of neuropsychiatric disease. RayBiotech mouse protein arrays were used to detect levels of 1308 proteins in serum and CSF. Fluorescence intensities from arrays were normalized to internal standards and controls. Fold change (FC) and multiple-test-corrected Mann Whitney p-values determined viable biomarkers (serum: FC > 1.5 or < 0.6 and p < 0.05; CSF: only p < 0.1). Pearson correlation identified serum proteins related to lpr behavioral testing scores (p < 0.05). DAVID software identified top gene ontology terms and KEGG pathways corresponding to biomarkers.
Results: 150 serum proteins distinguished between lupus mice and non-lupus controls (p < 0.05). Of these, 104 were increased and 46 were decreased in lupus mice (Figure 1A). Significant enrichment in cytokine signaling, apoptotic activity, and cellular immune responses was present (Figure 1B-D). Among those 150 proteins, 66 correlated with at least one feature of murine NPSLE (Figure 2A). From this subset, enrichment analyses showed involvement of cytokine signaling and innate immunity (Figure 2B-D). 27 CSF proteins distinguished between lupus mice and controls (increased in two or more lpr samples; p < 0.1), including several inflammatory cytokines and neuronal products (Figure 3A). Enrichment analyses identified inflammatory disease activity and cytokine signaling in the central nervous system (Figure 3B-D).
Conclusion: We succeeded in identifying novel serum biomarkers of murine NPSLE; several chemokines, cytokines, and neuronal proteins could facilitate systemic immune system targeting of the CNS. For the first time, a comprehensive picture of the MRL/lpr mouse CSF has been produced. Several inflammatory and neurotoxic cytokines are increased, indicating significant neuroinflammatory activity in lupus. In addition to finding possible diagnostic markers of NPSLE, these results also help clarify its mechanism and identify putative therapeutic targets.



Disclosures: J. Reynolds, None; C. Mohan, None; Y. Li, None; C. Putterman, Equillium, Progentec, Kidneycure.

