Back
Poster Session B
Reproductive health
Session: (0939–0969) Reproductive Issues in Rheumatic Disorders Poster
0956: Contribution of Antibody Titers/Specificities to Adverse Pregnancy Outcomes in a Multicenter Prospective Study of anti-Ro Positive Mothers
Sunday, November 13, 2022
9:00 AM – 10:30 AM Eastern Time
Location: Virtual Poster Hall
- NF
Nicola Fraser
NYU School of Medicine
New York, NY, United States
Abstract Poster Presenter(s)
Nicola Fraser1, Mala Masson2, Kristina Deonaraine3, Philip Carlucci4, Colin Phoon5, Ashley Roman5, Peter Izmirly6, Amit Saxena5, Michael Belmont6, Christina Penfield5, Young Mi Lee5, Julie Nusbaum7, Bruce Solitar5, Fardina Malik5, Paula Rackoff1, Rebecca Haberman1, Ruben Acherman8, Elena Sinkovskaya9, Alfred Albuhamad9, Majd Makhoul10, Gary Satou11, Nelangi Pinto12, Anita Moon-Grady13, Lisa Howley14, Stephanie Levasseur15, Jyothi Matta16, Christopher Lindblade17, Andrew Rubenstein18, Caitlin Haxel19, Katherine Kohari20, Joshua Copel20, James Strainic21, Tam Doan22, Karla Bermudez-Wagner22, Shreya Sunil Sheth22, Stacy Killen23, Theresa Tacy24, Michelle Kaplinski24, Bailey Drewes25, Robert Clancy6, Bettina Cuneo26 and Jill Buyon6, 1NYU School of Medicine, New York, NY, 2NYU Langone Medical Center- Division of Rheumatology, New York, NY, 3University at Buffalo, Buffalo, NY, 4NYU Langone, New York, NY, 5NYU Langone Health, New York, NY, 6NYU Grossman School of Medicine, New York, NY, 7NYU Long Island School of Medicine, Mineola, NY, 8Children's Heart Center, Las Vegas, 9East Virginia Medical School, Norfolk, VA, 10University of Kentucky, Lexington, KY, 11University of California Los Angeles, Los Angeles, CA, 12University of Utah, Salt Lake City, 13UCSF, San Francisco, 14Midwest Fetal Care Center, Children's Minnesota/Allina Health, Minneapolis, MN, 15Columbia University, New York, NY, 16University of Louisville, Louisville, KY, 17Phoenix Children's Hospital, Phoenix, 18Dignity Health, Phoenix, 19University of Vermont Children's Hospital, Burlington, VT, 20Yale University, New Haven, CT, 21UH Rainbow Babies, Cleveland, OH, 22Baylor College of Medicine, Houston, TX, 23Vanderbilt University, Nashville, TN, 24Stanford University, Stanford, CA, 25University of Colorado, Denver, CO, 26University of Colorado School of Medicine, Aurora, CO
Background/Purpose: While it is well accepted that congenital heart block (CHB) and neonatal annular rash are highly associated with maternal anti-Ro60 and 52 autoantibodies, the contribution of these auto-reactivities to other adverse pregnancy outcomes (APOs) is often discussed during pregnancy counseling with limited data available. Moreover, it is unknown whether titers or specificities of these putative antibodies alone or in the setting of additional maternal factors such as ethnicity, race, and/or health status influence APOs. Accordingly, this study was initiated to address these considerations leveraging a large multi-center prospective cohort of pregnancies (aimed at identifying emergent CHB) in which women were recruited solely on the basis of a known positive test for anti-Ro antibodies.
Methods: 216 pregnant patients recruited across the U.S. with anti-Ro antibodies present based on commercial testing at local laboratories were enrolled prior to 18 weeks gestation and prospectively followed. Anti-Ro antibody titers were confirmed at a central research laboratory by ELISA using native Ro60 and recombinant Ro52. All mothers provided self-reported demographic data, and maternal health status was assessed by clinical interviews and patient medical records. APOs reported to the DSMB included premature delivery < 37 weeks, small for gestational age defined as birth weight < 5th percentile, neonatal or fetal death > 12 weeks, pre-eclampsia at any time, and/or severe oligohydramnios. CHB was considered a separate outcome from APO.
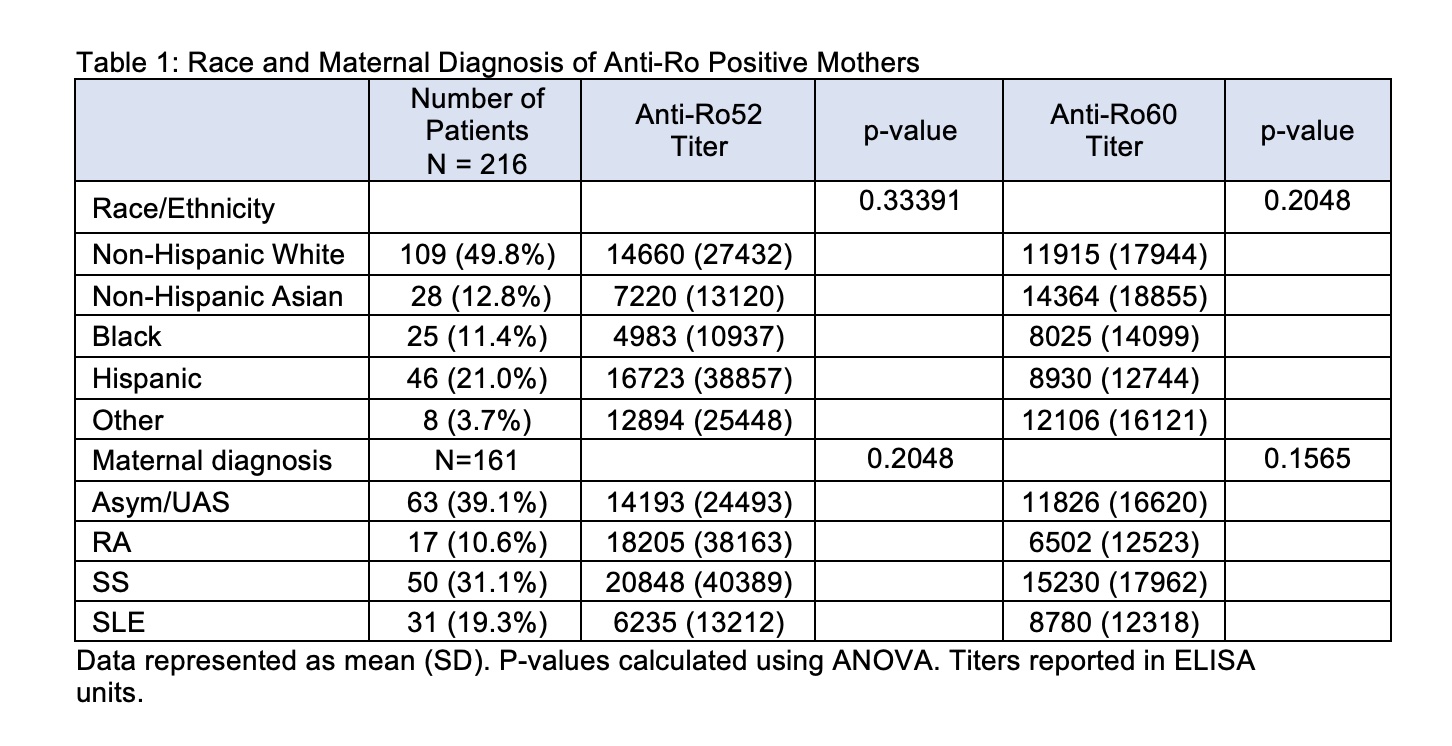
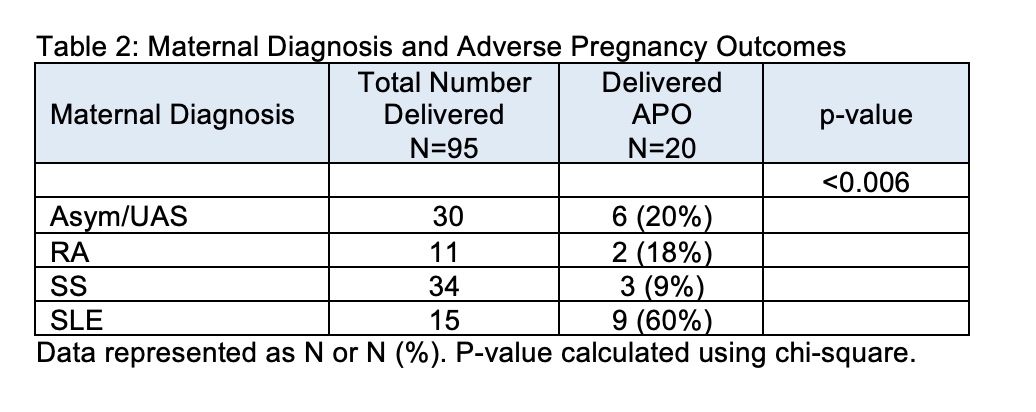
Results: Anti-Ro60 and anti-Ro52 titers were independent of race and ethnicity (Table 1). In addition, average titers did not differ by maternal diagnosis which was determined for 161 patients and included: asymptomatic/Undifferentiated Autoimmune Syndrome (UAS) (N = 63), Sjögren's Syndrome (SS) (N = 50), Systemic Lupus Erythematosus (SLE) (N = 31), and Rheumatoid Arthritis (RA) (N = 17) (Table 1). Of the enrolled patients, 109 completed pregnancies with APOs occurring in 20 (23%) and CHB occurring in 4 patients. Mean titers of anti-Ro60 and anti-Ro52 did not associate with APOs (mean +/- SD, anti-Ro60: no APOs 9404 +/- 13514; with APOs 10930 +/- 14412; p-value = 0.6537. anti-Ro52: no APOs 13690 +/- 31068; with APOs 21356 +/- 42203, p-value = 0.3610). In contrast to antibody titers and specificities, the maternal diagnosis did influence APOs. Mothers with established SLE were significantly more likely to have APOs than any of the other disease groups (P< 0.006) (Table 2).
Conclusion: The titer and specificity of anti-Ro antibodies did not segregate based on ethnicity, race, or maternal diagnosis. Moreover, APOs were not influenced by antibody titers or specificities, but rather by the maternal diagnosis of SLE.
Disclosures: N. Fraser, None; M. Masson, None; K. Deonaraine, None; P. Carlucci, None; C. Phoon, None; A. Roman, None; P. Izmirly, Momenta/Janssen; A. Saxena, None; M. Belmont, None; C. Penfield, None; Y. Lee, None; J. Nusbaum, None; B. Solitar, None; F. Malik, None; P. Rackoff, None; R. Haberman, None; R. Acherman, None; E. Sinkovskaya, None; A. Albuhamad, None; M. Makhoul, None; G. Satou, None; N. Pinto, None; A. Moon-Grady, None; L. Howley, None; S. Levasseur, None; J. Matta, None; C. Lindblade, None; A. Rubenstein, None; C. Haxel, None; K. Kohari, None; J. Copel, None; J. Strainic, None; T. Doan, None; K. Bermudez-Wagner, None; S. Sheth, None; S. Killen, None; T. Tacy, None; M. Kaplinski, None; B. Drewes, None; R. Clancy, None; B. Cuneo, None; J. Buyon, Equillium, GlaxoSmithKlein(GSK), L and M Healthcare Communications, Janssen, Boomcom, Merck/MSD.
Background/Purpose: While it is well accepted that congenital heart block (CHB) and neonatal annular rash are highly associated with maternal anti-Ro60 and 52 autoantibodies, the contribution of these auto-reactivities to other adverse pregnancy outcomes (APOs) is often discussed during pregnancy counseling with limited data available. Moreover, it is unknown whether titers or specificities of these putative antibodies alone or in the setting of additional maternal factors such as ethnicity, race, and/or health status influence APOs. Accordingly, this study was initiated to address these considerations leveraging a large multi-center prospective cohort of pregnancies (aimed at identifying emergent CHB) in which women were recruited solely on the basis of a known positive test for anti-Ro antibodies.
Methods: 216 pregnant patients recruited across the U.S. with anti-Ro antibodies present based on commercial testing at local laboratories were enrolled prior to 18 weeks gestation and prospectively followed. Anti-Ro antibody titers were confirmed at a central research laboratory by ELISA using native Ro60 and recombinant Ro52. All mothers provided self-reported demographic data, and maternal health status was assessed by clinical interviews and patient medical records. APOs reported to the DSMB included premature delivery < 37 weeks, small for gestational age defined as birth weight < 5th percentile, neonatal or fetal death > 12 weeks, pre-eclampsia at any time, and/or severe oligohydramnios. CHB was considered a separate outcome from APO.
Results: Anti-Ro60 and anti-Ro52 titers were independent of race and ethnicity (Table 1). In addition, average titers did not differ by maternal diagnosis which was determined for 161 patients and included: asymptomatic/Undifferentiated Autoimmune Syndrome (UAS) (N = 63), Sjögren's Syndrome (SS) (N = 50), Systemic Lupus Erythematosus (SLE) (N = 31), and Rheumatoid Arthritis (RA) (N = 17) (Table 1). Of the enrolled patients, 109 completed pregnancies with APOs occurring in 20 (23%) and CHB occurring in 4 patients. Mean titers of anti-Ro60 and anti-Ro52 did not associate with APOs (mean +/- SD, anti-Ro60: no APOs 9404 +/- 13514; with APOs 10930 +/- 14412; p-value = 0.6537. anti-Ro52: no APOs 13690 +/- 31068; with APOs 21356 +/- 42203, p-value = 0.3610). In contrast to antibody titers and specificities, the maternal diagnosis did influence APOs. Mothers with established SLE were significantly more likely to have APOs than any of the other disease groups (P< 0.006) (Table 2).
Conclusion: The titer and specificity of anti-Ro antibodies did not segregate based on ethnicity, race, or maternal diagnosis. Moreover, APOs were not influenced by antibody titers or specificities, but rather by the maternal diagnosis of SLE.


Disclosures: N. Fraser, None; M. Masson, None; K. Deonaraine, None; P. Carlucci, None; C. Phoon, None; A. Roman, None; P. Izmirly, Momenta/Janssen; A. Saxena, None; M. Belmont, None; C. Penfield, None; Y. Lee, None; J. Nusbaum, None; B. Solitar, None; F. Malik, None; P. Rackoff, None; R. Haberman, None; R. Acherman, None; E. Sinkovskaya, None; A. Albuhamad, None; M. Makhoul, None; G. Satou, None; N. Pinto, None; A. Moon-Grady, None; L. Howley, None; S. Levasseur, None; J. Matta, None; C. Lindblade, None; A. Rubenstein, None; C. Haxel, None; K. Kohari, None; J. Copel, None; J. Strainic, None; T. Doan, None; K. Bermudez-Wagner, None; S. Sheth, None; S. Killen, None; T. Tacy, None; M. Kaplinski, None; B. Drewes, None; R. Clancy, None; B. Cuneo, None; J. Buyon, Equillium, GlaxoSmithKlein(GSK), L and M Healthcare Communications, Janssen, Boomcom, Merck/MSD.

